
Purification and enzymatic characteristics of cysteine desulfurase, IscS, in Acidithiobacillus ferrooxidans ATCC 23270
WU An-na(吴安娜), ZHANG Yan-fei(张燕飞), ZHENG Chun-li(郑春丽), DAI Yun-jie(戴云杰),
LIU Yuan-dong(刘元东), ZENG Jia(曾 嘉), GU Guo-hua(顾帼华), LIU Jian-she(柳建设)
Key Laboratory of Biometallurgy of Ministry of Education, School of Minerals Processing and Bioengineering,
Central South University, Changsha 410083, China
Received 20 September 2008; accepted 5 November 2008
Abstract: A cysteine desulfurase protein, IscS, was encoded by the operon iscSUA in Acidithiobacillus ferrooxidans. The gene of IscS from Acidithiobacillus ferrooxidans ATCC 23270 was cloned and expressed in Escherichia coli. The protein was purified by one-step affinity chromatography to homogeneity. The final protein yield after affinity chromatography was 12.9%. The enzyme was characterized for thermal stability, pH and kinetic parameters. The molecular mass of recombinant IscS was 46 ku by SDS-PAGE. The optimum pH was 8.0-8.5. The enzyme had a temperature optimum at 30 ℃ and was relatively stable at 40 ℃, with 67% loss of activity. 1,5-I-AEDANS significantly inhibited IscS activity. Kinetic parameters Km and Vmax were found to be 0.11 mmol/L and 2.57 μmol/(L?min).
Key words: Acidithiobacillus ferrooxidans; IscS; purification; optimum pH; optimum temperature; inhibition; kinetics
1 Introduction
Iron-sulfur proteins are widely distributed in almost all organisms and play essential roles in energy metabolism, DNA repair, transcriptional regulation, and biosynthesis of nucleotides and amino acids[1-2]. Three separate systems including ISC (iron-sulfur cluster) [3], NIF(nitrogen fixation)[4], and SUF(sulfur)[5] have been identified for bacterial Fe-S clusters biosynthesis. Of these systems, ISC appears to provide a fundamental Fe-S cluster biosynthesis pathway. The isc operon (iscRSUA-hscBA-fdx) encoded the components of the ISC machinery, which are conserved from bacteria to higher eukaryotes and, in Escherichia coli[6].
Three isc genes, iscS, iscU and iscA, have been the targets of recent investigations. IscU is characterized as an Fe-S cluster assembly scaffold protein[7-8]. The function of IscA remains controversial in the biogenesis of Fe-S proteins. It might function as an iron donor or an alternate scaffold for Fe-S clusters synthesis[9-10]. IscS is a cysteine desulfurase which is a pyridoxal 5′- phosphate(PLP)-dependent enzyme. IscS catalyzes the conversion of L-cysteine to L-alanine and sulfan sulfur via the formation of a protein-bound cysteine persulfide intermediate on a conserved cysteine residue, which is the major cellular catalyst for the mobilization and distribution of sulfur from cysteine for a number of different biosynthetic pathways[3, 11-12].
Acidithiobacillus ferrooxidans is a motile, Gram- negative, chemolithotrophic bacterium that utilizes Fe(Ⅱ), H2S ,S0, reduced inorganic sulfur compounds and molecular hydrogen as energy sources. The oxidation of sulfur produces sulfuric acid which is responsible for some of the interesting characteristics of At. ferrooxidans. The presence of sulfuric acid strongly acidifies the environment. Therefore, At. ferrooxidans is an acidophile by necessity, growing within a pH range of 1.3-4.5. It is generally found in acidic environments such as mining dumps and acid mine drainages. This organism plays a key role of microbial communities taking part in the bacterial-chemical processes of bioleaching under mesophilic conditions.
2 Experimental
2.1 Materials
Acidithiobacillus ferrooxidans ATCC 23270 was obtained from the American Type Culture Collection. A HiTrap chelating metal affinity column was purchased from GE healthcare LTD. Top10 competent cells, E. coli strain BL21(DE3) competent cells came from Invitrogen Life Technologies. The Plasmid Mini kit, a gel extraction kit and synthesized oligonucleotides were obtained from Sangon Company of Shanghai, China. L-cysteine, PLP, Taq DNA polymerase, T4 DNA ligase and restriction enzymes came from MBI Fermentas, Germany. 1,5-I-AEDANS, N-Ethylmaleimide N,N-dimethyl-p- phenylenediamie, and D-cysteine came from Sigma. All other reagents were of research grade or better and were obtained from commercial sources.
2.2 Cloning of ISCS gene from A. ferrooxidans ATCC 23270
Genomic DNA from A. ferrooxidans was prepared using the EZ-10 spin column genomic DNA isolation kit from Bio Basic Inc., according to the manufacturer’s instructions for bacterial DNA extraction. This genomic DNA was used as a template for PCR reaction. The gene was amplified by PCR using primers that were designed to add six continuous histidine codons to the 5′ primer. The sequence of the forward primer was 5′-CGCGCGA- ATTCAGGAGGAATTTAAAATGAGAGGATCGCATCACCATCACCATCACATCGAGGGAAGGTTGCGGATCGATCCGGATCGTCCCATTTAT-3′, containing an EcoRI site (GAATTC), a ribosome binding site (AGGAGGA), codons for the amino acid sequence MRGSHHHHHH (start codon and hexahistag), and codons for amino acids 2-11 of mature IscS. The sequence of the reverse primer was 5′-CTGCAGGGAT- CCTTAGTGCGCGGCCCACTGAATGCTGTGGATA-TCAAT-3′, containing a BamHI site (GGATCC), a stop anticodon(TTA), and anticodons for the last 11 amino acids of mature IscS protein. PCR amplification was performed using Taq DNA polymerase, and samples were subjected to 25 cycles of 45 s of denaturation at 94 ℃, 1 min of annealing at 65 ℃, and 2 min of elongation at 72 ℃. The amplification products were analyzed by electrophoresis on a 1% agarose gel and stained with ethidium bromide. The resulting PCR product was gel purified, double digested and ligated into a pLM1 expression vector, resulting in the pLM1::IscS plasmid [13]. The identified positive colony was grown in LB medium containing ampicillin (50 mg/L), and the plasmid pLM1:IscS was isolated from harvested bacteria cells using a plasmid extraction kit. The isolated pLM1::IscS plasmid was then transformed into E. coli strain BL21 (DE3) competent cells for expression purposes.
2.3 Expression of soluble recombinant protein IscS in E. coli
We use the following procedure for expression of soluble IscS protein. The E. coli strain BL21(DE3) cells with pLM1::ISCS plasmid were grown at 37 ℃ in 500 mL of LB medium containing ampicillin (100 mg/L) to an optical density at 600 nm (OD600) of 0.6. At this point, the cells were incubated at room temperature with the addition of 0.5 mmol/L isopropyl-D-thiogalactopyrano- side (IPTG) overnight with shaking at 180 r/min. The cells were harvested by centrifugation and the cell pellet was washed with an equal volume of sterile water. The cells were again harvested by centrifugation, suspended in start buffer (20 mmol/L potassium phosphate, pH 7.4, 0.5 mol/L NaCl), incubated with 5 mg lysozyme at room temperature for 0.5 h, and then stored at -80 ℃ for purification.
2.4 Purification of A. ferrooxidans ATCC23270 IscS
The cells were lysed by sonication four times for 30 s each time (150 W Autotune Series High Intensity Ultrasonic sonicator equipped with a 8 mm diameter tip). The insoluble debris was removed by centrifugation and the clear supernatant was used for protein purification. A Hi-Trap column (6E Healthcare Ltd) was first equilibrated with 0.1 mol/L nickel sulfate to charge the column with nickel ions followed by 5 column volumes of MiliQ water to remove unbound nickel ions from the column, and then by 5 column volumes of start buffer (20 mmol/L potassium phosphate, pH 7.4, 0.5 mol/L NaCl) to equilibrate the column. The clarified sample was applied to the Hi-Trap column after filtering it through a 0.45 μm filter. The column was washed with 5 column volumes of start buffer followed with 5 column volumes of wash buffer (20 mmol/L potassium phosphate, pH 7.4, 0.5 mol/L NaCl, 50 mmol/L imidazole), and subsequently the protein was eluted with an elution buffer (20 mmol/L potassium phosphate, pH 7.4, 0.5 mol/L NaCl, 500 mmol/L imidazole). The method of Bradford was used to determine the protein content with BSA as standard. We used the SDS-PAGE with 15% (volume fraction) acrylamide to analyze the eluted fractions[14]. The gels were stained with Coomassie Brilliant Blue R-250. The purified enzyme fractions were combined and dialyzed against a 20 mmol/L potassium phosphate buffer, pH 7.4, 5% (volume fraction) glycerol, and then stored at -80 ℃.
2.5 UV-Vis scanning
Ultraviolet-visible spectra scanning was carried out at 25 ℃ on a Techcomp UV-2300 spectrophotometer. The samples of IscS (10 μmol/L) were prepared in 20 mmol/L phosphate buffer containing 0.5 mol/L NaCl, pH 7.4. L-Cysteine (1.0 mmol/L) and D-Cysteine (1.0 mmol/L) were added in two samples of wild-type IscS separately and incubated for 30 min. Blank sample was also performed on other identical solutions lacking cysteine.
2.6 Assay of A. ferrooxidans IscS activity
The activity of A.ferrooxidans IscS was determined by measuring the rate of production of sulfide from L-Cysteine incubated under anaerobic conditions in l mL of 50 mmol/L Tris-HCl (pH 8.0), 10 mmol/L MgCl2, 1.0 mmol/L L-Cysteine, 5 mmol/L dithiothreitol (DTT), and the sample containing the enzymatic activity. The incubations were carried out in autosampler vials sealed at the start of the incubation with Teflon-lined caps. Control assays were also performed on other identical solutions lacking cysteine.
Then we used the following method to determine the sulfide concentration. First, 100 μL of 30 mmol/L FeCl3 in 1.2 mol/L HCl were added to the vial to stop the reaction. Second, 100 μL of 20 mmol/L N,N-dimethyl-p- phenylenediamine dihydrochloride in 7.2 mol/L HCl was added to generate the methylene blue. After mixing, the test tube was incuated for 30 min at room temperature in the dark. Samples were centrifuged to remove solid sulfur, and spectrophotometric measurements of the methylene blue formed were done at 670 nm. A calibration curve had been made previously using Na2S [11]. As this method does not take account of the loss of sulfide through reactions with sulfur, the activities determined are underestimated.
2.7 Enzyme kinetics
2.7.1 Effect of pH and temperature
The IscS activity reaction was taken in different pH values from 5.5 to 10.2. Appropriate buffers (0.2 mol/L acetic acid, pH 5; 0.2 mol/L phosphate, pH 5-7 and 0.05 mol/L Tris-HCl, pH 7-10) were used to determine the optimum pH. The optimum pH values obtained from this assay were used in all the other experiments.
The effect of temperature on IscS activity was measured within 10-50 ℃. We used temperature controlled water bath to reach these temperatures.
2.7.2 Thermal inactivation
Heat treatments of IscS wild type were carried out at 35, 45, 55, 65 and 75 ℃ for varied periods of time in a temperature controlled water bath; 0.5 mL of enzyme solution was placed in a pre-warmed tube at the specified temperature and 0.01 mL liquor was withdrawn at various time intervals, cooled and assayed for residual activity. The stability of the enzyme was expressed as percentage of residual activity and was calculated by comparison with untreated enzyme.
2.7.3 Effect of inhibitors
N-ethylmaleimide, 1,5-I-AEDANS, allylglycine, SDS and urea were evaluated for their effectiveness as inhibitors of IscS activity, using the sulfide production assay. The results were reported as percentage of L-cysteine inhibition.
2.7.4 Determination of Km and vmax
IscS activity was assayed in buffers at optimum pH value and ambient temperature. The Km value and maximum velocity vmax were determined by the Lineweaver-Burk plot[15].
2.8 Molecular structure modeling of IscS from A. ferrooxidans ATCC23270
A preliminary model for the structure of IscS was constructed using the approach of comparative protein modeling, applied by the Modeller module of Insight II software (Accelrys software Inc.) running on Dell Precision 470 workstation with Redhat Linux system. The predicted protein structure was carried out on the basis of the sequences of the IscS from A. ferrooxidans and E. coli, because their sequence identity is relatively high (66.2%). The template structure of the IscS from E. coli (PDB entry code 1P3W) refined to 2.3 ? was used for modeling. We used the GenDoc Software for sequence alignment of template and target sequences.
3 Results and discussion
3.1 Cloning of A. ferrooxidans ATCC 23270 IscS gene
PCR technique was used to successfully add six continuous histidine residues to the N-terminal of the IscS from A. ferrooxidans ATCC 23270, which greatly accelerated the protein purification process. The transformant was grown in LB medium and the corresponding plasmid was isolated and re-transformed to E. coli BL21(DE3) for expression.
3.2 Expression and purification of IscS from A. ferrooxidans ATCC 23270
Optimum expression of IscS protein from A. ferrooxidans ATCC 23270 in E. coli BL21(DE3) was conducted with 0.5 mmol/L IPTG at room temperature (25 ℃) rather than at 37 ℃. Induced cells showed a characteristic intense yellow color due to the presence of the pyridoxal 5′-phosphate (PLP) cofactor in the overproduced cysteine desulfurase.
Nickel metal-affinity resin column was used for single-step purification of His-tagged IscS protein. The wild-type IscS protein also had an intense yellow color, due to the presence of the PLP cofactor in the purified protein. The yellow color was also reported in other IscSs from E. coli, B.pertussis, and Geobacillus stearothermophilus V[3, 16-17].
The final protein yield after affinity chromatography was 12.9%, which suggests that the T7 polymerase promoter/BL21(DE3) expression system is an ideal system for expressing the IscS in high yield. The purified protein were dialyzed overnight against a potassium phosphate buffer, pH 7.4, 5% glycerol as soon as possible after the purification. The addition of 5% glycerol was essential for maintaining the long-term stability of the protein. The purities of the purified IscS was further examined by SDS-PAGE and single band corresponding to the 46 ku protein was observed with >95% purity (Fig.1). The wild-type protein could be stored at 4 ℃ for 2 months without significant change of activity.

Fig.1 Coomassie blue-stained SDS-PAGE of purified IscS from A. ferrooxidans ATCC23270: Lane 1—Molecular mass standards; Lane 2—Purified IscS
The protein purification is shown in Table 1. The final yield from the purification, based on the desulfurization of L-cysteine to give hydrogen sulfide, was 39%, and the total activity of purified IscS was 1 475.4 nm of hydrogen sulfide produced per minute, and the specific activity was 148 nmol of hydrogen sulfide produced per minute per milligram of protein (Table 1).
Table 1 Purification summary of IscS from A. ferrooxidans ATCC 23270

3.3 UV-visible light scanning of IscS
The UV-visible spectrum of the recombinant IscS is shown in Fig.2. The wild-type IscS belonged to a family of PLP-dependent enzymes and exhibited the UV-visible spectrum characteristic of other cysteine desulfrases, with an absorbance maximum for PLP centered at (420±1) nm. The spectra were similar to those previously reported for the IscS and NifS from various sources.
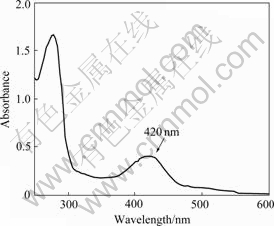
Fig.2 UV-visible absorbance spectrum of purified IscS from A. ferrooxidans ATCC23270 (Characteristic absorption peak of PLP is indicated by arrowhead)
The results of incubation of L-cysteine and D-cysteine with IscS separately for 10 min are very distinct. The optical spectrum of IscS exhibited a decrease in absorbance at (416±1) nm which was incubated in the presence of L-cysteine. However, there was no obvious change on the spectrum in the addition of D-cysteine. The results suggested that the L-cysteine was the only substrate for this enzyme and the PLP participated in the cysteine desulfuration reaction. Our IscS activity assay in which the substrate L-cystein was replaced by D-cysteine has the identic results (data not show). All the results were also in agreement with the previous reports[3, 17-18].
3.4 Enzyme kinetics
3.4.1 Effect of pH and temperature
The pH optimum for enzyme-catalyzed desulfuration of L-cysteine in various buffers was found to occur at pH 8.0-8.5. At pH above 10.0 or below 5.0, the activity decreased very rapidly. This value was close to the optimal pH of IscS from Geobacillus stearothermophilus V[11]. The effect of pH on cysteine dusulfurase activity is shown in Fig.3. The rapid inactivation of the enzyme above pH 8.0 or below 5.0 may be due to conformational changes in the enzyme under the acidic or alkaline conditions.
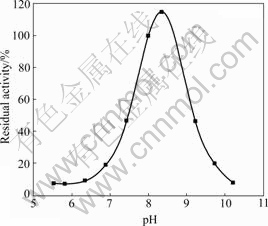
Fig.3 Effect of pH on IscS activity
The activity of IscS was measured at different temperatures at optimum pH for 2 h. The enzyme showed the highest activity at 30 ℃. The optimal temperature was different from the Geobacillus stearothermophilus V IscS[18]. Nevertheless, the optimum temperature of 30 ℃ was the same as the optimum growth temperature of Acidithibacillus ferrooxidans. The effect of temperature on cysteine dusulfurase activity is shown in Fig.4.

Fig.4 Effect of temperature on IscS activity
3.4.2 Thermal inactivation kinetics
The enzyme was incubated at different temperatures (35-65 ℃) for 2 h and, after cooling, the residual enzyme activity was measured (Fig.5). The residual activity of the enzyme exceeded 95% when it was incubated at 35 ℃ for 2 h. However, the residual activity of the enzyme was only 33% when it was incubated at 40 ℃ for 2 h. The deactivation was faster when incubated at 55 ℃; no activity occurred virtually after 30 min. Consequently, enzyme was stable at 35 ℃ but unstable at temperatures above 55 ℃. The decrease in percentage of residual activity at higher temperatures is due to the unfolding of the tertiary structure.
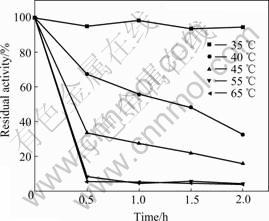
Fig.5 Thermal stability of IscS at varied temperatures (35-65 ℃) versus time
3.4.3 Effect of inhibitors
The effects of various inhibitors on the purified IscS are shown in Table 2. The results were reported as percentage of L-cysteine inhibition. It was markedly inhibited by 1,5-I-AEDANS. N-ethylmaleimide, allylglycine and urea showed minimum inhibition. N-ethylmaleimide was reported to be a strong inhibitor of NIFS[11]. It was also reported that L-lylglycine was a membrane permeable suicide inhibitor on IscS, which forms covalent adduct with the active site cysteine of IscS in E. coli, resulting in enzyme inactivation[12].
Table 2 Effect of inhibitors on IscS activity

If the IscS was incubated under anaerobic conditions in the presence of 5 mmol/L dithiothreitol, and then the the reduced enzyme was incubated with equal amounts of 1,5-I-AEDANS, the inhibition of IscS was up to 90%. However, the inhibition of the other inhibitors had no obvious change after the same operation. The reciprocal of the amount of 1,5-I-AEDANS required to inactivate 1 mol IscS was measured. Results of typical experiment are presented in Fig.6. The result of a tryptic digest prepared from the inactivated protein indicated that the cysteine (Cys 331) was modified by 1,5-I-AEDANS[3,19]. Clearly, the cysteine residue in IscS participates in the desulfurization reaction in the presence of L-cysteine.

Fig.6 Effect of 1,5-I-AEDANS on IscS activity with and without prior incubation with dithiothreitol (Protein was assayed for activity after treatment with 1,5-I-AEDANS using sulfide production assay)
3.4.4 Km and vmax of IscS from A.ferrooxidans ATCC 23270
Km and vmax values of cysteine desulfurase IscS were calculated from the Lineweaver–Burk graphs and the results are shown in Table 3 and Fig.7. The Km for IscS from A. ferrooxidans ATCC 23270 was 0.11 mmol/L and vmax was 2.57 μmol/(L?min). The result shows that the substrate-binding site of IscS has a high affinity for L-cysteine.
Table 3 Detection results for Km and Vmax of cysteine desulfurase IscS


Fig.7 Km and vmax of cysteine desulfurase IscS (Lineweaver– Burk)
3.5 Molecular modeling of IscS from A. ferrooxidans
Among all the available molecular structures of IscS, the IscS from E. coli was identified as the best structural template for modeling the molecular structure of IscS from A. ferrooxidans ATCC 23270. This model was refined by MM optimization and MD simulation.
The modeled overall structure of IscS from A. ferrooxidans ATCC 23270 was shown to be a monomer(Fig.8). Sequence alignment for IscS from various sources showed that the five residues His106, Lys208 and Cys331 were highly conserved residues(Fig.9). It is reported that the lysinyl (Lys208 in A. ferrooxidans ATCC 23270) and cysteinyl (Cys331 in A. ferrooxidans ATCC 23270) residues are considered to be the probable PLP-binding and active-site cysteinyl residues [11,17-18]. Our molecular modeling of IscS from A.ferrooxidans also supports these points: the PLP group interacted with Lys208 by forming an internal aldimine Schiff base; the imidazole ring of His106 was positioned directly above the pyridine ring of PLP, which was likely to function as an acid-base catalyst in several protonation/ deprotonation steps during catalysis. So His106 was also significant for the catalytic reaction. Therefore, we will choose these residues for site-directed mutagenesis to study their functions for the catalytic reaction in the next investigation.
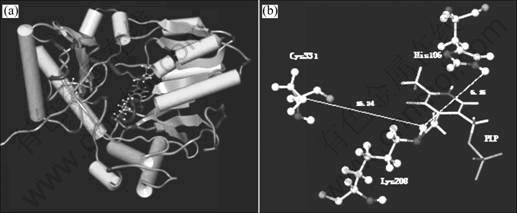
Fig.8 Overall structure of IscS from A. ferrooxidans ATCC 23270 (a) and modeled IscS structure (b) showing active site

Fig.9 Sequences alignment of IscS from A.ferrooxidans and other sources (Sequence alignment was made by GenDoc software): A.ferrooxidans—IscS from Acidithiobacillus ferrooxidans ATCC 23270; B.vietnamiensis—IscS from Burkholderia vietnamiensis G4; R.metallidurans—IscS from Ralstonia metallidurans CH34; D.acidovorans—IscS from Delftia acidovorans SPH-1; E.coli—IscS from Escherichia coli; M.flagellatus—IscS from Methylobacillus flagellatus KT; L.cholodnii—IscS from Leptothrix cholodnii SP-6; A.succinogenes—IscS from Actinobacillus succinogenes 130 Z. Some important residues which are conserved are marked with *.
4 Summary
We report here the first overexpression of E.coli of His-tagged IscS protein from A. ferrooxidans ATCC23270. There are partial characteristics of IscS: the optimum reaction temperature of cysteine desulfurase was 30 ℃, the optimum reaction pH was 8.0-8.5. The enzyme was stable at 35 ℃ but unstable at temperatures above 55 ℃. The inhibiting effect of inhibitors was 1,5-I-AEDANS >SDS >N-ethylmaleimide> allylglycine>urea. The Km for IscS from A. ferrooxidans ATCC 23270 was 0.11 mmol/L and vmax was 2.57 μmol/ (L?min).
Abbreviations
Abbreviations used are as follows: A. ferrooxidans, Acidithiobacillus ferrooxidans; Amino acids; C, Cys, cysteine; H, His, histidine; K, Lys, lysine; PLP, pyridoxal5′-phosphate-dependent; 1,5-I-AEDANS, N-iodoacety- N′-(5-sulfo-1-naphthay)ethylenedialmine; IPTG, isopropyl-D-thiogalactopyranoside; DTT, dithiothreitol; IscA, iron-sulfur cluster assembly protein A; IscS, iron-sulfur cluster assembly protein S; IscU, iron-sulfur cluster assembly protein U; PAGE, polyacrylamide gel electrophoresis; PCR, polymerase chain reaction; SDS, sodium dodecylsulfate; UV/Vis, ultraviolet-visible spectroscopy.
References
[1] REES D C, HOWARD J B. The interface between the biological and inorganic worlds: Iron-sulfur metalloclusters [J]. Science, 2003, 300: 929-931.
[2] Fontecave M. Iron-sulfur clusters: Everexpanding roles [J]. Nat Chem Biol, 2006, 2: 171-174.
[3] ZHENG L, CASH V L, FLINT D H, DEAN D R. Assembly of iron-sulfur clusters: Identification of an iscSUA-hscBA-fdx gene cluster from Azotobacter vinelandii [J]. J Biol Chem, 1998, 273: 13264-13272.
[4] TOKUMOTO U, ITAMURA S, UKUYAMA K, TAKAHASHI Y. Interchangeability and distinct properties of bacterial Fe-S cluster assembly systems: Unfunctional replacement of the isc and suf operons in Echerichia coli with the nifSU-like operon from Helicobacter pylori [J]. J Biochem, 2004: 99-209.
[5] BARRAS F, LOISEAU L, PY B. How Escherichia coli and Saccharomyces cerevisiae build Fe/S proteins [J]. Adv Microb Physiol, 2005, 50: 41-101.
[6] SCHWARTZ C J, GIEL J L, PATSCHKOWSKI T, LUTHER C, RUZICKA F J, BEINERT H, KILEY P J. IscR, an Fe-S cluster-containing transcription factor, represses expression of Escherichia coli genes encoding Fe-S cluster assembly proteins [J]. Proc Natl Acad Sci USA, 2001, 98: 14895-14900.
[7] YOSHIMITSU S, KEI W, KEIICHI F. The asymmetric trimeric architecture of [2Fe-2S] IscU: Implications for its scaffolding during iron–sulfur cluster biosynthesis [J]. J Mol Biol, 2008, 383: 133-143.
[8] YANG J, BITOUN J P, DING H. Interplay of IscA and IscU in biogenesis of iron-sulfur clusters [J]. J Biol Chem, 2006, 281: 27956-27963.
[9] DING H, CLARK R J, DING B. IscA mediates iron delivery for assembly of iron-sulfur clusters in IscU under the limited accessible free iron conditions [J]. J Biol Chem, 2004, 279: 37499-37504.
[10] KOZO M, EIKI Y, YOUHEI K, SOO J L. The asymmetric IscA homodimer with an exposed [2Fe-2S] cluster suggests the structural basis of the Fe-S cluster biosynthetic scaffold [J]. J Mol Biol, 2006, 360: 117-132.
[11] ZHENG L, WHITE R H, CASH V L, JACK R F, DEAN D R. Cysteine desulfurase activity indicates a role for NifS in metallocluster biosynthesis [J]. Proc Natl Acad Sci USA, 1993, 90: 2754-2758.
[12] ZHENG L, WHITE R H. Mechanism for the desulfurization of L-Cysteine catalyzed by the nifS gene product [J]. Biochemistry, 1994, 33: 4714-4720.
[13] SODEOKA M, LARSON C, CHEN L, LAND W, VERDINE G. A multifunctional plasmid for protein expression by ECPCR: Overproduction of the p50 subunit of NF-KB [J]. Bioorg Med Chem Lett, 1993, 3: 1095-1100.
[14] LAEMMLI U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4 [J]. Nature, 1970, 227: 680-685.
[15] LINEWEAVER H, BURK D. The determination of enzyme dissociation constant [J]. J Amer Chem Soc, 1934, 56: 658-661.
[16] CUPP-VICKERY J R, URBINA H, VICKERY L E. Crystal structure of IscS, a cysteine desulfurase from Escherichia coli [J]. J Mol Biol, 2003, 330: 1049-1059.
[17] FLINT D H. Escherichia coli contains a protein that is homologous in function and n-terminal sequence to the protein encoded by the nifS Gene of Azotobacter vinelandii and that can participate in the synthesis of the Fe-S cluster of dihydroxy-acid dehydratase [J]. J Biol Chem, 1996, 271: 16068-16074.
[18] YANG W, ROGERS P A, DING H. Repair of nitric oxide modified ferredoxin [2Fe-2S] cluster by cysteine desulfurase (IscS) [J]. J Biol Chem, 2002, 277: 12868-12873.
[19] TANTALE?N J C, ARAYA M A. The Geobacillus stearothermophilus V iscS gene, encoding cysteine desulfurase, confers resistance to potassium tellurite in Escherichia coli K-12 [J]. J Bacteriol,2003, 185: 5831-5837.
Foundation item: Project(2004CB619204) supported by the National Basic Research Program of China; Project(50621063) supported by the National Natural Science Foundation of China; Project(200549) supported by the Foundation of National Excellent Doctroal Dissertation of China
Corresponding author: LIU Jian-she, Tel: +86-13917301289; E-mail: ljscsu@263.net
(Edited by PENG Chao-qun)