Physical, chemical, and surface charge properties ofbauxite residue derived from a combined process
来源期刊:中南大学学报(英文版)2019年第2期
论文作者:杜平 任杰 陈娟 郭伟 杨宾 秦小鹏
文章页码:373 - 382
Key words:bauxite residue; surface charge properties; metals; chemical species; leaching behavior
Abstract: A detailed understanding of the composition, buffering capacity, surface charge property, and metals leaching behavior of bauxite residue is the key to improved management, both in reducing the environmental impact and using the material as an industrial by-product for other applications. In this study, physical, chemical, and surface charge properties of bauxite residue derived from a combined process were investigated. Results indicated that the main alkaline solids in bauxite residue were katoite, sodalite, and calcite. These minerals also lead to a higher acid neutralizing capacity of bauxite residue. Acid neutralizing capacity (ANC) to pH 7.0 of this residue is about0.9 mol H+/kg solid. Meanwhile, the Fe-, Al-, and Si-containing minerals in bauxite residue resulted in an active surface; The isoelectric point (IEP) and point of zero charge (PZC) were 7.88 and 7.65, respectively. This also leads to a fact that most of the metals in bauxite residue were adsorbed by these surface charged solids, which makes the metals not readily move under natural or even moderately acidic conditions. The leaching behavior of metals as a function of pH indicated that the metals in bauxite residue present low release concentrations (pH > 3).
Cite this article as: REN Jie, CHEN Juan, GUO Wei, YANG Bin, QIN Xiao-peng, DU Ping. Physical, chemical, and surface charge properties of bauxite residue derived from a combined process [J]. Journal of Central South University, 2019, 26(2): 373–382. DOI: https://doi.org/10.1007/s11771-019-4009-7.

ARTICLE
J. Cent. South Univ. (2019) 26: 373-382
DOI: https://doi.org/10.1007/s11771-019-4009-7
REN Jie(任杰)1, 2, CHEN Juan(陈娟)2, GUO Wei(郭伟)1,YANG Bin(杨宾)2, QIN Xiao-peng(秦小鹏)2, DU Ping(杜平)2
1. Ministry of Education Key Laboratory of Ecology and Resource Use of the Mongolian Plateau &
Inner Mongolia Key Laboratory of Environmental Pollution Control and Waste Resource Recycle,
School of Ecology and Environment, Inner Mongolia University, Hohhot 010021, China;
2. State Key Laboratory of Environmental Criteria and Risk Assessment, Chinese Research Academy of Environmental Sciences, Beijing 100012, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract: A detailed understanding of the composition, buffering capacity, surface charge property, and metals leaching behavior of bauxite residue is the key to improved management, both in reducing the environmental impact and using the material as an industrial by-product for other applications. In this study, physical, chemical, and surface charge properties of bauxite residue derived from a combined process were investigated. Results indicated that the main alkaline solids in bauxite residue were katoite, sodalite, and calcite. These minerals also lead to a higher acid neutralizing capacity of bauxite residue. Acid neutralizing capacity (ANC) to pH 7.0 of this residue is about 0.9 mol H+/kg solid. Meanwhile, the Fe-, Al-, and Si-containing minerals in bauxite residue resulted in an active surface; The isoelectric point (IEP) and point of zero charge (PZC) were 7.88 and 7.65, respectively. This also leads to a fact that most of the metals in bauxite residue were adsorbed by these surface charged solids, which makes the metals not readily move under natural or even moderately acidic conditions. The leaching behavior of metals as a function of pH indicated that the metals in bauxite residue present low release concentrations (pH > 3).
Key words: bauxite residue; surface charge properties; metals; chemical species; leaching behavior
Cite this article as: REN Jie, CHEN Juan, GUO Wei, YANG Bin, QIN Xiao-peng, DU Ping. Physical, chemical, and surface charge properties of bauxite residue derived from a combined process [J]. Journal of Central South University, 2019, 26(2): 373–382. DOI: https://doi.org/10.1007/s11771-019-4009-7.
1 Introduction
Bauxite residue is a by-product in the manufacture of alumina extracted from bauxite ores. Globally, the inventory of bauxite residue reached about 3.5 billion tons in 2014 with the speed of 120 million tons per annum [1]. These residues are generally deposited in nearby engineered impoundments [2]. The high alkalinity and sodic content are the primary reasons for classification of bauxite residue as a hazardous material. This property also causes the formation of friable dust-prone surfaces and might contribute to the failure of embankment [3]. Therefore, the bauxite residue disposal area was susceptible to wind and water erosion and can be a potential contamination source to surrounding environments. In addition, the toxic trace metals are also known to be present in the bauxite residue, and pose potential environmental risk to resource utilization and ecological disposal [4].Resource utilization and ecological disposal of bauxite residue is of environmental, ecological, and economic significance [5, 6]. Bauxite residue derived from differing ore sources and refining processes varied in physic, chemical, and mineralogical properties. Most alumina enterprises in the world use the Bayer process to produce alumina, and therefore the physicochemical characteristics of the Bayer process bauxite residue are the research field that most scholars pay attention to [7]. However, there are approximately 30 alumina enterprises in China, and very few alumina Co. used the Bayer Process [8]; Annually 70 million tons of bauxite residue are produced from the combined process, and the accumulated inventory of bauxite residue has reached an estimated 0.6 billion tons [9]. The combined process is the principal industrial means of producing alumina in China since 98% of bauxite mines are medium to low grade and contain a high proportion of Si. During the Bayer-sintering combined process, the bauxite containing a high proportion of Si was firstly conducted to a caustic leach through Bayer process, and then the resulting bauxite residue containing sodium aluminum silicates was sintered with limestone and soda ash [10].
The alkaline nature of bauxite residue makes it a potential acid-neutralizing agent, and researchers found that bauxite residue also has a strong binding capacity for heavy metals and phosphates [11, 12]. The ecological disposal and environmental applications of the Bayer process bauxite residue have received substantial research [13–15], but little has been done to bauxite residue derived from the combined process. Therefore, this study was conducted to determine the physical, chemical, and surface charge properties of the combined process bauxite residue. The objectives were: 1) to characterize bauxite residue derived from a combined process; 2) to determine the fractionation of metals in bauxite residue; and 3) to analyze the leaching behavior of metals from bauxite residue.
2 Materials and methods
2.1 Basic theory
The bauxite residue (BR) samples were collected from the bauxite residue disposal area (BRDA) of the State Power Investment, China. The bauxite residue was produced from a combined process. The combined bauxite residue samples were obtained from a depth of 0–20 cm in the BRDA, and transported to the laboratory, air dried at room temperature, and passed through a 2-mm sieve before use.
2.2 Physical, chemical, and mineralogical analyses
The pH and EC of bauxite residue were measured in a solid–water suspension at a ratio of 1:2.5 and 1:5, respectively. Exchangeable sodium percentage (ESP) was calculated as 100× exchangeable Na/CEC, and the CEC was determined by the sum of exchangeable Na, K, Ca, and Mg. They were extracted with 1 mol/L ammonium acetate (pH 7) and analyzed by ICP-MS (7500 ICP-MS, Agilent Technologies, USA) [16, 17]. Total alkalinity (TA) was the sum of the [CO32–] and [HCO3–], and they were determined by titration [18]. The particle size of the bauxite residue was determined by a laser particle size analyzer (LA 950, Horiba, Japan). The microspore analyzer (ASAP 2020 HD 88, Micrometrics, USA) was used to determine the surface area of bauxite residue. Acid neutralizing capacity (ANC) of bauxite residue was determined by rapid titrating. The bauxite residue suspension (5 g bauxite residue mixed with 25 mL deionized water in a 500 mL conical flask) was titrated with 0.1 mol/L HNO3/NaOH to pH 2–13. After each titration, the conical flask was sealed with a sealing film and place in a horizontal shaker for 24 h to ensure pH stable.
Before the mineral composition, micromorphology and microstructure were determined, the bauxite residue sample was dried at 65 °C for 48 h and lightly crushed to a fine powder using an agate mortar. They were determined by using X-ray diffractometer (XRD, D8 Advance, Bruker, USA), scanning electron microscope (S480; Hitachi, Japan), and electron probe micro-analyzer (EPMA, JXA 8230, JEOL, Japan), respectively.
2.3 Surface electrochemical properties
The bauxite residue contains a large number of free bases and soluble salts. It is necessary to remove these ions for eliminating the influence on the electrokinetic and surface charge determination. The flushing method proposed by FREIRE et al [19] was used to remove the dissolved substances in bauxite residue. 5 g of bauxite residue and 30 mL of Milli-Q water was placed in 50 mL centrifuge tubes and centrifuged at 4500 r/min for 10 min and the supernatant were decanted. Repeat the washing step until the pH and EC of the supernatant were below 8.0 and 60 μS/cm, respectively. After washing, the sample was dried at 65 °C for 48 h and passed through a 125-μm sieve for determination of the surface charge and electrokinetic.
The surface charge of the bauxite residue was measured by acid-base potentiometric titration in a 0.01 mol/L and 0.1 mol/L potassium nitrate electrolyte solution. Bauxite residue through a 125-μm sieve (0.02 g) was added to 100 mL electrolyte of varying concentration (0.01 mol/L and 0.1 mol/L KNO3) and equilibrated under an N2 atmosphere, in a water bath with controlled temperature at 25 °C, until the change in pH (ΔpH) over 5 min was less than 0.01. The equilibrated bauxite residue suspensions were then slowly titrated at increments of 10–100 μL by adding 0.1 mol/L HNO3 or 0.1 mol/L KOH to endpoint of pH 10 and 3.5, respectively. Record the corresponding pH and addition amount of HNO3 and KOH until the ΔpH is less than 0.01 within 5 min after each titration. The electrolyte without the bauxite residue sample as the blank, and titrations of blank solutions were conducted with the same procedure. Two replicates were performed for each set of experiments.
Bauxite residue through a 125-μm sieve (0.1 g) was added to 100 mL electrolyte of varying concentration (0.001 mol/L and 0.01 mol/L KNO3) and equilibrated under an N2 atmosphere, in a water bath controlled temperature at 25 °C, until the change in pH (△pH) over 5 min was less than 0.01. A similar titration process was used by the endpoints of pH 2 and 10, respectively. The suspensions were obtained for zeta potential analysis (Zetasizer Nano ZS, Malvern) after ultrasonic dispersion for 5 min. Zeta potential as a function of pH was plotted and the isoelectric point (IEP) can be defined as the pH at which electrophoretic mobility is zero.
2.4 Fractionation and leaching behavior of metals in bauxite residue
Fractionation of metals in bauxite residue was determined using a sequential extraction procedure based on a scheme of the European Community Bureau of Reference and targeted the acid-soluble, reducible, oxidizable, and residual fractions. Suspensions from each step were centrifuged, filtered, and analyzed for metal contents.
The pH dependent leach test is designed to characterize the leaching behavior of metals in bauxite residue as a function of pH (US EPA 2009a, Method 1313). It has been proven that this leach test is one of the most useful methods to characterize the behavior of metals under different exposure conditions [20]. The pH dependent leach test was conducted by leaching the bauxite residue samples over a pH range of 2–13 at a liquid–solid ratio (L/S) of 10 mL/g (addition of HNO3 or KOH was based on the ANC titration procedure). The suspensions were shaken for 48 h at 30 r/min, and centrifuged at 3000 r/min for 15 min and filtered using a PES syringe filter (pore size of 0.22 μm). The metal contents for each extraction procedure were measured by ICP-MS.
3 Results and discussion
3.1 Characterization of bauxite residue
3.1.1 Basic physicochemical property
The bauxite residue samples have been described previously [21] and are summarized in Table 1. Na, K, Ca, Mg, Al, and Fe are the major elements in bauxite residue, with an order of Ca>Na>Al>Fe>Mg~K. The content of minor metals in bauxite residue is relatively low, such chemical composition different from previously reported for the Bayer processed bauxite residue [6]. The trace metals in the Bayer process bauxite residue had concentrations of Cr 620–1350 mg/kg, As 32–213 mg/kg, V 860–963 mg/kg, Cd 1.3 mg/kg, Ni 80 mg/kg, Pb 50 mg/kg, and Zn 80 mg/kg [22]. The pH, total alkalinity (TA) and ESP of bauxite residue were 11.21, 16.56 mg/kg, and 23.93%, respectively. Such chemical properties are similar to previously reported bauxite residue [6]. A large number of nitrate and sulfate were found in bauxite residue. In fact, bauxite residue also contains more carbonate. However, due to the slow release of alkaline solids in bauxite residue, the determination of carbonate concentration by conventional titration methods results in much lower than the actual concentration. Research has found that at least 50 d was required for the complete release of alkaline in bauxite residue [23]. The bauxite residue particle size ranges from 0.9 to 68 μm with an average of 9.1 μm; it is therefore in the fine sand textural class.
Table 1 Selected chemical and physical properties of bauxite residue sample
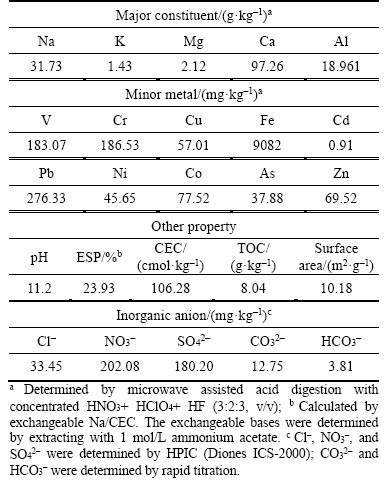
Other research also reported that the particle size of bauxite residue averages 2 to 200 μm with a typical range of 100 nm to 200 μm [24].
3.1.2 Mineral composition
The mineralogical phases present in the bauxite residue were identified by XRD (Figure 1). The complex components were contained in bauxite residue, and the main minerals were katoite, sodalite, calcite, and hematite. The main minerals that caused bauxite residue to be alkaline are katoite, sodalite, and calcite. KONG et al [25] indicated that the alkaline phases account for 44.3% of the total solid phase in bauxite residue. With the low solubility and slow alkaline release rate, these alkaline solids lead to a continuously alkaline release, and increase of pH and concentration of Al(OH)4–, CO32–, HCO3–, and OH– to the system. The alkaline solids also provide buffering capacity for bauxite residue and these solids together, leading to bauxite residue with a strong ability to prevent pH from change.
3.2 Bauxite residue alkalinity and associated chemical properties
3.2.1 Mineral composition
The ANC was used to assess the quantity of acid needed to neutralize the bauxite residue. It included slow acid–base reactions with multiple solid phases [26]; meanwhile, the ANC can also measure the amount of inorganic acid required to reach a specific pH endpoint [27]. The ANC of bauxite residue was determined by the rapid titration, and the corresponding curve in Figure 2 indicated that the initial pH of the bauxite residue in the titration was 11.2, and the ANC to pH 7.0 is about 0.55 mol H+/kg soil. Multiple inflection points on ANC curve indicate a complete solid dissolution and a new phase as a buffer.
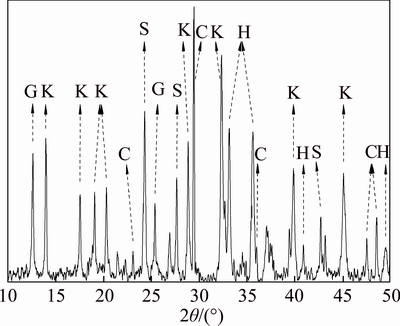
Figure 1 XRD pattern of bauxite residue: G–Gobbinsite, Na4Ca(Si10Al6)O32·12H2O; K–Katoite, Ca3Al2(SiO4)·(OH)8; S–Sodalite, (Na6Al6Si6O24)·2Na2SO4; C–Calcite, CaCO3; H–Hematite, Fe2O3
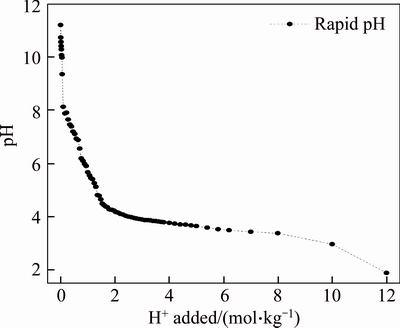
Figure 2 Acid neutralization capacity of bauxite residue
The length of the plateau is related to the amount of buffer [26]. Researches have also found that the desiliconization products began to dissolve at pH 6.3 and it completely disappeared at pH 5.4; The calcite exhibited the greatest buffering capacity near pH 4.5; The complete dissolution of cancrinite, calcite, and andradite was at an abrupt inflection point near pH 3.0 [6, 26]. The buffering curve at pH of 2–3 may attribute to the dissolution of Fe-oxides, such as amorphous ferrihydrite and hematite.
3.2.2 Micromorphological
The bauxite residue micromorphology determined by EPMA indicated that the element of Na is uniformly distributed in small particles in bauxite residue, and Ca is distributed in larger granular particles (Figure 3). Meanwhile, the primary constituents in bauxite residue particles were Ca and Al oxides. A high consistency degree of Ca—C distribution indicated that Ca is mainly combined with carbonate in bauxite residue; High consistency degree of Na—Ca and Na—Al distribution indicated that Na was likely to be adsorbed by calcium and aluminum compounds; The consistency of the Na and S distributions may show the presence of sodium sulfide; High consistencies of Al–O and Fe–O distributions were also observed. These results are consistent with the analysis of XRD.
3.2.3 Surface electrochemical
Particles larger than 1 nm and less than 2 μm are usually called colloidal particles. Colloid is the most active part of physical and chemical properties, related to the phenomenon of ion adsorption and exchange, aggregation and dispersion, and buffering properties [16, 28]. Bauxite residue is a mixture of heterogeneous solids with a fine structure, and to a great extent, the behavior between particles depends on the surface chemistry of the constituent particles. Therefore, understanding the mineral surface charge properties is of significance to the adsorption and agglomeration of the bauxite residue. The bauxite residue contains a large number of silicate minerals and inorganic components such as Fe, Al, Si oxides and hydroxides mineral. These minerals are the main solid phase part of the surface active in bauxite residue [28, 29]. In general, the surface composed of the inorganic component of a clay including the surface consisting of a clay particle unit includes the silicone tetrahedral sheet connected siloxane surface, the alumina octahedron piece connected surface, —Si—OH, and —Al—OH composed surface. According to the results of XRD analysis, the above structure is also ubiquitous in bauxite residue particles. These surfaces carry different charges to together form a colloidal surface with different charge species, charge quantity, and charge distribution.
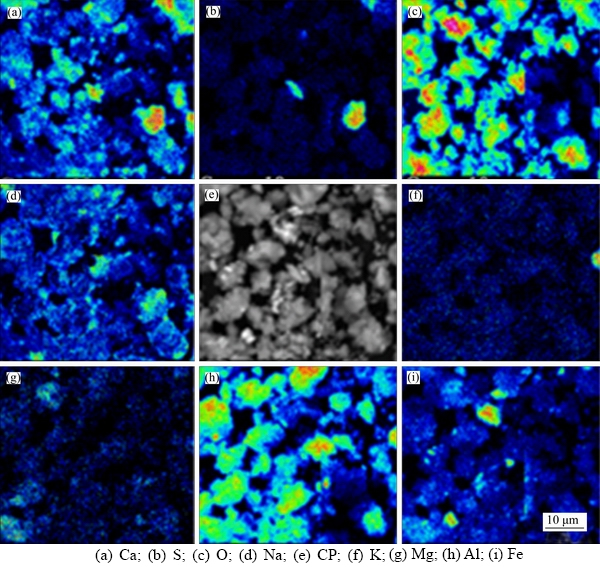
Figure 3 Elemental micro-area distribution analysis of bauxite residue:
The phase composition of bauxite residue mainly includes iron oxides, aluminum oxides, calcium-containing minerals, and silicon oxides. The results of mineralogical analysis further confirm that the bauxite residue mineral composition is complex, and Fe, Al, Ca, Ti and Si appear among the different minerals, including hematite, calcite, gobbinsite, katoite, and sodalite. The chemical composition and mineral composition of these minerals in bauxite residue reveal that there are both pH-related variable charges in the residue, such as oxides or minerals of Fe, Al, Ti and Si, as well as permanent charges independent of pH, such as one of the desiliconized products of the cancrinite (isomorphic replacement of Si, Al and Fe).
The interface electromotive force, also known as the zeta potential, is an important indicator of variable charge, reflecting the stability of the system. In this study, zeta potential of bauxite residue was determined in 0.001 mol/L and 0.010 mol/L KNO3 electrolyte solution with pH ranging from 2 to 10. The result is shown in Figure 4. The curve intersection point of the two concentrations of electrolyte solution is the isoelectric point (IEP); it indicates the corresponding system pH value at which the electrolyte concentration does not affect the surface charge. The bauxite residue has an isoelectric point of 7.88, which is similar to the IEP of bauxite residue reported in other research at 6.35–8.70 [28]. However, at IEP, the zeta potential of bauxite residue is negative and exhibits a slight negative charge, probably caused by the adsorption of ions by the variable charge surface of the bauxite residue particles. The particles with a high zeta potential improved the particle dispersion. It is generally considered that the zeta potential is from 30 to–30 mV, which is the dividing line between particle dispersion and stability. As a mixture of various minerals and oxides, electrokinetic properties bauxite residue are determined by the electrokinetic properties of these mineral and oxide components.
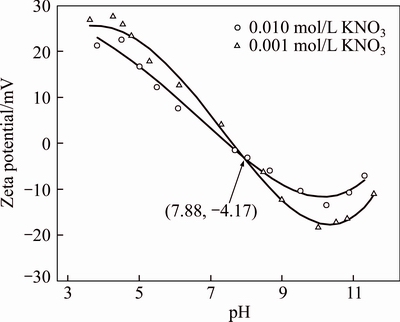
Figure 4 Electrokinetic properties of bauxite residue
In order to quantify the contribution of each component to the bauxite residue electrokinetic properties, the relationship between the total IEP of the mixture and the individual component IEP is as follows [30]: IEP=∑Fi×IEPi, where Fi is the content of a component, %; IEP is the isoelectric point of the corresponding component. Mineral species of the common charge sources were Fe2O3, Al2O3, SiO2, CaO, and TiO2. The IEP of mixture was calculated based on their respective contents and IEP. The oxide composition of the bauxite residue used in this study was 16.51% Fe2O3, 28.11% Al2O3, 16.88% SiO2, 26.4% CaO, and 1.14% TiO2. The corresponding IEP was 8.37, 8.03, 2.2, 12.3, and 5.6, respectively [22, 24]. The calculated isoelectric point of the mixture is 7.32, and the difference between the bauxite residue isoelectric points obtained by experimental test was 0.56. The calculated IEP was slightly lower than the measured IEP, which may be attributed to ignorance of some low levels of oxides, such MgO, V2O5, and Cr2O3. It also can be seen that the minerals containing these five oxides are the main source of charge in the bauxite residue.
Usually, the hydrated oxide surface is a variable charge surface and the positive, negative and the amounts of charges carried by it, depend on the pH of the oxide and the water soluble system. When the sum of the surface charges of all sources is equal to 0, the positive and negative charge densities in the system are equal, and the pH of the corresponding system is pHPZC. The point of zero charge (PZC) is an important surface parameter of hydrated oxide surface. When the system pH < PZC, the surface of the oxide is positively charged. When the system pH>pHPZC, the surface is negatively charged. At this time, non-specific adsorption occurred. When there is no specific adsorption in the system, IEP=PZC.
The PZC of bauxite residue was determined by the same method using 0.10 mol/L and 0.01 mol/L KNO3 electrolyte solution, and the result is shown in Figure 5. The intersection point of the curves of different electrolyte solutions is PZC, as shown in Figure 5; the pHPZC of bauxite residue is about 7.65, which is slightly lower than IEP. The possible reason is the existence of specific adsorption. After the oxide surface in bauxite residue specifically adsorbed the metal cations, the cations accumulation in the stern layer leads to an increase in the adsorption of OH– on the surface, which requires a higher H+ concentration to re-maintain the electrical neutrality of the oxide surface, causing the PZC decrease and IEP increase. In addition, the existence of Si—OH can also promote the surface electronegativity of the products adsorbed by each other. After co-precipitation of iron oxides and aluminum oxides, this led to an increase in H+ to achieve electrical neutralization, and therefore PZC decreased.
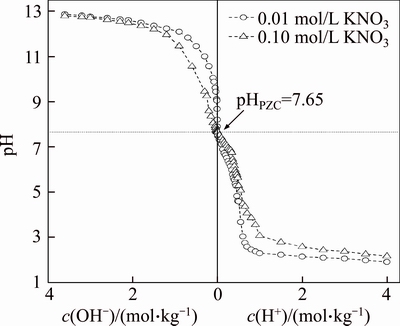
Figure 5 PZC titration curve of bauxite residue
3.3 Chemical species and leaching behavior of metals in bauxite residue
3.3.1 Fractionation
The chemical species of metals in bauxite residue samples were determined to study the metals transformation and bioavailability. The metals in bauxite residue were mainly residual fractions (Figure 6). This indicated that most of the metals in bauxite residue were stable under natural conditions. Research has also found that the metals in bauxite residue remaining in not readily mobile fractions are likely to associate with poorly reactive phases and are strongly fixed in bauxite residue [22, 31]. The fraction of potentially mobile (e.g. acid soluble, reducible and oxidizable fractions) metals was low for all the metals (< 15%), with the exception of V, Cr, and Cu. The fractionation for V, Cr, and Cu showed that the potentially mobile fractions accounted for 33%, 25%, and 22% of the total, respectively. A significant fraction of V, Cr, and Cu could be released by moderate acidification. However, the bauxite residue contains only 183 mg/kg V, 186 mg/kg Cr, and 57 mg/kg Cu, respectively, as well as a strong acid buffering capacity, which together result in a small potential release of these metals. Except the residual fractions, the major chemical species of Cd, Pb, Ni, and Co were oxidizable fractions.
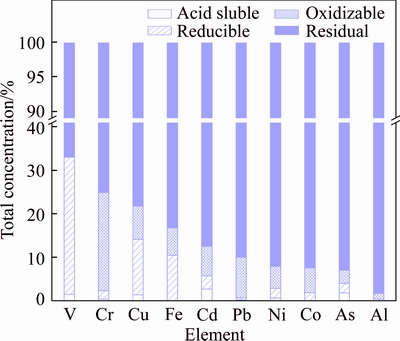
Figure 6 Fractionation of metals in bauxite residue
3.3.2 Leaching behavior
To understand the leaching behavior of metals in bauxite residue, the response of relationship of the metals behavior in bauxite residue as a function of pH was studied (Figure 7). As can be seen, the minimum dissolved metals appear at different pH titration endpoints. The minimum leaching concentration of Cd and Pb was at pH 4; Al, Cr, and V were at pH 5.5; As, Ni, and Co were at pH 9; Fe and Cu were at pH 10. Leaching concentrations of Cd and Pb significantly increased when the pH is lower than 4, but the leaching concentration keeps essentially constant over the pH ranges of 4–13, which indicated that the Cd and Pb in bauxite residue were mainly associated with the oxides and hydroxides of Fe/Mn/Al, consistent with the BCR analysis and other research [22]. Behavior of Al, Cr, and V presented a highest release in the acidic and alkaline systems. The lowest leaching concentration in the neutral system was found in these metals leaching behavior, which may be due to their fractions in bauxite residue. Al mainly exists in the form of gibbsite and aluminum hydroxide, which are compounds that are highly pH dependent, they are most stable at pH 5.3–8.1, and dissolved quickly when the pH<5 of pH>10 [32]. Cr and V in bauxite residue may be mainly adsorbed by cancrinite and amorphous aluminum hydroxide [33]. The release behavior of As, Ni, Co and Fe, Cu in bauxite residue is similar in different environmental conditions. The leaching concentrations of these metals were all decreased with pH increasing, which might be due to a fact that the Fe/Mn bound metals and hydroxide were the main forms of their species. Taking together, obtained results show that the risk of toxic metals in bauxite residue seems to be small.
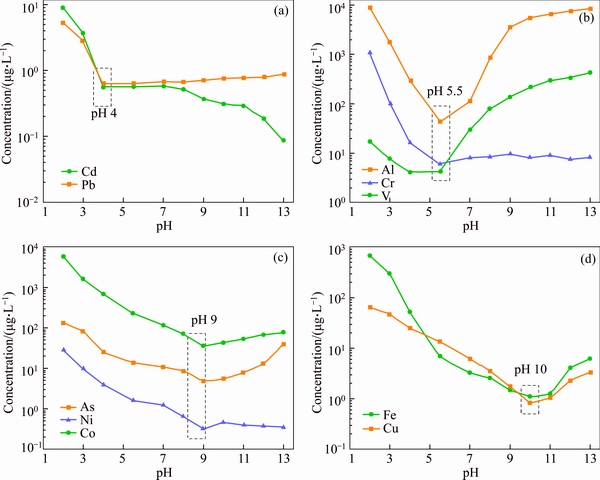
Figure 7 Response of relationship of metals behavior in bauxite residue as a function of pH
4 Conclusions
The bauxite residue mineral composition, alkalinity and associated chemical properties, and metal leaching behavior were investigated. The composition of the bauxite residue is not only important with respect to pH buffering, but also with respect to how surface charge and electric double layer thickness change. The main solid phases were katoite, sodalite, calcite, and hematite, which lead to a strong buffering capacity of 0.9 mol H+/kg bauxite residue at pH 7. The IEP and pHPZC of bauxite residue were 7.88 and 7.65, respectively. The bauxite residue contains significant concentrations of metals, some of which have a large content. The fractionation study showed that metals in bauxite residue are mainly associated with the residue phases, suggesting that they are not readily mobile under natural or even moderately acidic conditions. The leaching behavior of metals as a function of pH indicated that the metals in bauxite residue present low release concentrations, but the release concentration is drastically promoted under strong acidic conditions (pH<3). The leaching concentration of Al, Cr, and V drastically increased in strong alkaline conditions. The potential release of metals from bauxite residue could be higher than expected, due to enough acid needed to exhaust its neutralization capacity.
References
[1] POWER G, GRAFE M, KLAUBER C. Bauxite residue issues: I. Current management, disposal and storage practices [J]. Hydrometallurgy, 2011, 108(1, 2): 33–45.
[2] SCHMALENBERGER A, O'SULLIVAN O, GAHAN J, COTTER P D, COURTNEY R. Bacterial communities established in bauxite residues with different restoration histories [J]. Environmental Science & Technology, 2013, 47(13): 7110–7119.
[3] REN J, CHEN J, HAN L, WANG M, YANG B, DU P, LI F S. Spatial distribution of heavy metals, salinity and alkalinity in soils around bauxite residue disposal area [J]. Science of the Total Environment, 2018, 628–629: 1200–1208.
[4] BURKE I T, MAYES W M, PEACOCK C L, BROWN A P, JARVIS A P, GRUIZ K. Speciation of arsenic, chromium, and vanadium in red mud samples from the Ajka spill site, Hungary [J]. Environmental Science & Technology, 2012, 46(6): 3085–3092.
[5] XUE S G, WU Y J, LI Y W. Industrial wastes applications for alkalinity regulation in bauxite residue: A comprehensive review [J]. Journal of Central South University, 2019, 26(2): 268–288.
[6] LIAO J X, JIANG J, XUE S G, QING Y C, WU H, MANIKANDAN R, HARTLFY W, HUANG L B. A novel acid-producing fungus isolated from bauxite residue: The potential to reduce the alkalinity [J]. Geomicrobiology Journal, 2018, 35(10): 840–847.
[7] GRAFE M, POWER G, KLAUBER C. Bauxite residue issues: III. Alkalinity and associated chemistry [J]. Hydrometallurgy, 2011, 108(1, 2): 60–79.
[8] LIU W C, CHEN X Q, LI W X, YU Y F, YANG K. Environmental assessment, management and utilization of red mud in China [J]. Journal of Cleaner Production, 2014, 84: 606–610.
[9] XUE S G, ZHU F, KONG X F, WU C, HUANG L, HUANG N, HARTLFY W. A review of the characterization and revegetation of bauxite residues (Red mud) [J]. Environmental Science and Pollution Research, 2016, 23(2): 1120–1132.
[10] XUE S G, KONG X F, ZHU F, et al. Proposal for management and alkalinity transformation of bauxite residue in China [J]. Environmental Science and Pollution Research, 2016, 23(13): 12822–12834.
[11] HUA Y, HEAL K V, FRIESL-HANL W. The use of red mud as an immobiliser for metal/metalloid-contaminated soil: A review [J]. Journal of Hazardous Materials, 2017, 325: 17–30.
[12] LIANG Z, PENG X, LUAN Z, LI W H, ZHAO Y. Reduction of phosphorus release from high phosphorus soil by red mud [J]. Environmental Earth Sciences, 2011, 65(3): 581–588.
[13] SANTINI T C, MALCOLM L I, TYSON G W, WARREN L. pH and organic carbon dose rates control microbially driven bioremediation efficacy in alkaline bauxite residue [J]. Environmental Science & Technology, 2016, 50(20): 11164–11173.
[14] ZHU F, LIAO J X, XUE S G, HARTLEY W, ZOU Q, WU H. Evaluation of aggregate microstructures following natural regeneration in bauxite residue as characterized by synchrotron-based X-ray micro-computed tomography [J]. Science of the Total Environment, 2016, 573: 155–163.
[15] XUE S G, LI M, JIANG J, MILLAR G J, LI C X, KONG X F. Phosphogypsum stabilization of bauxite residue: Conversion of its alkaline characteristics [J]. Journal of Environmental Sciences, 2019, 77: 1–10. DOI: https:// doi.org/10.1016/j.jes.2018. 05.016.
[16] ZHU F, HOU J T, XUE S G, WU C, WANG Q L, HARTLEY W. Vermicompost and gypsum amendments improve aggregate formation in bauxite residue [J]. Land Degradation & Development, 2017, 28(7): 2109–2120.
[17] ZHU F, CHENG Q Y, XUE S G, LI C X, HARTLEY W, WU C, TIAN T. Influence of natural regeneration on fractal features of residue microaggregates in bauxite residue disposal areas [J]. Land Degradation & Development, 2018, 29(1): 138–149.
[18] LI X F, YE Y Z, XUE S G, JIANG J, WU C, KONG X F, HARTLEY W, LI Y W. Leaching optimization and dissolution behavior of alkaline anions in bauxite residue [J]. Transactions of Nonferrous Metals Society of China, 2018, 28(6): 1248–1255.
[19] FREIRE T S, CLARK M W, COMARMOND M J, PAYNE T E, REICHELT-BRUSHETI A J, THOROGOD G J. Electroacoustic isoelectric point determinations of bauxite refinery residues: different neutralization techniques and minor mineral effects [J]. Langmuir: the ACS Journal of Surfaces and Colloids, 2012, 28(32): 11802–11811.
[20] SLOOT H A V D. Harmonisation of leaching L extraction procedures for sludge, compost, soil and sediment analyses [M]//QUEVAUVILLER P. Methodologies for soil and sediment fractionation studies. The Royal Society of Chemistry, 2002: 142–174.
[21] REN J, LIU J D, CHEN J, LIU X L, LI F S, DU P. Effect of ferrous sulfate and nitrohumic acid neutralization on the leaching of metals from a combined bauxite residue [J]. Environmental Science and Pollution Research, 2017, 24(10): 9325–9336.
[22] RUBINOS D A, BARRAL M T. Fractionation and mobility of metals in bauxite red mud [J]. Environmental Science and Pollution Research, 2013, 20(11): 7787–7802.
[23] KHAITAN S. In-situ neutralization of stored bauxite residue [D]. Kansas State University, 2006.
[24] FULLER R D, NELSON E D P, RICHARDSON C J. Reclamation of red mud (bauxite residues) using alkaline-tolerant grasses with organic amendments [J]. Journal of Environmental Quality, 1982, 11(3): 533–539.
[25] KONG X F, LI M, XUE S G, HARTLEY W, CHEN C G, WU C, LI X F, LI Y W. Acid transformation of bauxite residue: Conversion of its alkaline characteristics [J]. Journal of Hazardous Materials, 2017, 324: 382–390.
[26] KHAITAN S, DZOMBAK, D A, LOWRY, G V. Chemistry of the acid neutralization capacity of bauxite residue [J]. Environmental Engineering Science, 2009, 26(5): 873–881.
[27] KONG X F, GUO Y, XUE S G, HARTLFY W, WU C, YE Y Z, CHENG Q Y. Natural evolution of alkaline characteristics in bauxite residue [J]. Journal of Cleaner Production, 2017, 143: 224–230.
[28] LIU Y, NAIDU R, MING H. Surface electrochemical properties of red mud (bauxite residue): Zeta potential and surface charge density [J]. Journal of Colloid and Interface Science, 2013, 394: 451–457.
[29] XUE S G, YE Y Z, ZHU F, WANG W L, JIANG J, HARTLEY W. Changes in distribution and microstructure of bauxite residue aggregates following amendments addition [J]. Journal of Environmental Sciences, 2019, 78: 276–286 DOI: https://doi.org/10.1016/j.jes.2018.10.010.
[30] PARK K, STUMM W. [CHAIRMAN], GOULD R F. Equilibrium concepts in natural water systems [J]. Limnology & Oceanography, 1967, 12(4): 726–727.
[31] SANTINI T C, PENG Y G. Microbial fermentation of organic carbon substrates drives rapid pH neutralization and element removal in bauxite residue leachate [J]. Environmental Science & Technology, 2017, 51(21): 12592–125601.
[32] LI Y W, JIANG J, XUE S G, MILLAR G J, KONG X F, LI X F, LI M, LI C X. Effect of ammonium chloride on leaching behavior of alkaline anion and sodium ion in bauxite residue [J]. Transactions of Nonferrous Metals Society of China, 2018, 28(10): 2125–2134.
[33] KOSMULSKI M. Compilation of PZC and IEP of sparingly soluble metal oxides and hydroxides from literature [J]. Advances in Colloid and Interface Science, 2009, 152(1, 2): 14–25.
(Edited by YANG Hua)
中文导读
赤泥的物理、化学和表面电荷特性研究
摘要:对赤泥组成、缓冲能力、表面电荷性质以及金属浸出行为的深入理解是促进赤泥管理的关键,既可以减少其对环境的影响,也可以将其作为工业副产品进行资源化利用。本文采用联合法处理赤泥,对赤泥的理化性质和表面电荷特性进行研究。结果表明,造成赤泥碱性的固体主要是加藤石、方钠石和方解石;到pH 7.0时,赤泥酸中和能力约为0.9 mol H+/kg;同时,赤泥含铁、铝和硅的矿物质使赤泥具有活性表面,赤泥的等电点和电荷零点分别为7.88和7.65。这也导致赤泥中的大多数金属被这个表面带电荷的固体所吸附,也导致金属在自然条件下,或者温和的酸性条件下较为稳定。在不同pH体系金属的浸出特性研究结果表明赤泥中大多数金属的释放浓度较低(pH>3)。
关键词:赤泥;表面电荷特性;金属;化学形态;浸出行为
Foundation item: Projects(41501350, 41461071, 31860170) supported by the National Natural Science Foundation of China
Received date: 2018-11-10; Accepted date: 2018-12-15
Corresponding author: DU Ping, PhD, Associate Professor; Tel: +86-10-84928849; E-mail: duping@craes.org.cn; ORCID: 0000-0001- 5100-0519

