J. Cent. South Univ. (2012) 19: 2440-2446
DOI: 10.1007/s11771-012-1294-9
Leaching process of selenium residue
LI Qian(李倩)1, ZHANG Bao(张宝)1, MIN Xiao-bo(闵小波)1, SHEN Wen-qian(申文前)2
1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. The First Detachment, Tianjin Contingent of Chinese Armed Police Forces, Tianjin 300222, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract: A thermodynamics analysis on the leaching process of selenium residue and discussion on the behaviors of aqueous ionic in the leaching process were carried out. Through thermodynamical calculation, the values of  and relevant potential expressions were obtained. According to these thermodynamical data, the φ-pH diagrams of Se-H2O system at 298 and 373 K were obtained; Simultaneously, the φ-pH diagrams of SO2-H2O and SO2-Se-H2O systems were drawn at 298 K. With increasing the temperature, the stable regions of
and relevant potential expressions were obtained. According to these thermodynamical data, the φ-pH diagrams of Se-H2O system at 298 and 373 K were obtained; Simultaneously, the φ-pH diagrams of SO2-H2O and SO2-Se-H2O systems were drawn at 298 K. With increasing the temperature, the stable regions of  ,
,  and
and  in the φ-pH diagram of Se-H2O system become gradually large, but the limits of pH in the stable region become gradually small. The stability area of reduction precipitation in the SO2-Se-H2O system was finally determined. The results of oxidization leaching experiments of selenium residue indicate that when the mass ratio of selenium residue to sodium chlorate is 2, the concentration of sulfuric acid is 300 g/L and the residue is agitated for 3 h at leaching temperature of 80 °C, the leaching rate of selenium could reach 97.76 %. The experimental results conform the calculated results by φ-pH diagram. The selenium reduction precipitation in oxidization-leaching solution was analyzed under the conditions of acidity of 150 g/L, the sodium sulphite concentration of 35 g/L at the reductive temperature of 23 °C for 120 min. And this demonstrates the thermodynamics analysis.
in the φ-pH diagram of Se-H2O system become gradually large, but the limits of pH in the stable region become gradually small. The stability area of reduction precipitation in the SO2-Se-H2O system was finally determined. The results of oxidization leaching experiments of selenium residue indicate that when the mass ratio of selenium residue to sodium chlorate is 2, the concentration of sulfuric acid is 300 g/L and the residue is agitated for 3 h at leaching temperature of 80 °C, the leaching rate of selenium could reach 97.76 %. The experimental results conform the calculated results by φ-pH diagram. The selenium reduction precipitation in oxidization-leaching solution was analyzed under the conditions of acidity of 150 g/L, the sodium sulphite concentration of 35 g/L at the reductive temperature of 23 °C for 120 min. And this demonstrates the thermodynamics analysis.
Key words: thermodynamics analysis; selenium residue; Se-H2O system; φ-pH diagram; SO2-Se-H2O system
1 Introduction
Selenium is the rare element associated with natural sulfur and relatively high complex ability with some heavy or noble metals [1-3]. Most of selenium ore has few industrial exploitation values due to the scarce of metallogenic ore. However, selenium has many and continuously increasing industrial applications used in solar cell, photoelectric camera tube, and metallic addition [4-6] in spite of serious shortage shortcoming. Raw materials for extraction of selenium which have been extensively studied by several researchers in pyrometallurgical processes are copper anode slime [7-9], lead blast furnace flue dust, residual mud of sulfuric acid factory, steelmaking dust, lead-zinc concentrate ore roasting flue dust, pyrite roasting residue [10-11] etc. But these methods have some defects: long flow process, low recovery of selenium, impurity of selenium powder, serious environmental pollution etc; finally, further purification such as vacuum-distillation and oxidization-reduction [12-14] have to conduct. On the other hand, mercury and gold production waste are generated in the hydrometallurgical process of selenium extraction [10]. The constituent elements, and their concentrations, depend on the characteristics of the raw materials that are processed, the species and their concentration, the reactor type used in the different stages of the process and the conditions under which these stages operate. All these factors affect the chemical and mineralogical characteristics of selenium residue, thus explaining their variability and, consequently, the processes chosen for their treatment, which is based on the recovery of valuable elements and the stabilization of undesirable elements present in the residue. In recent years, the development of recycling selenium technology which is based on hydrometallurgical separation such as extraction [15], ion exchange [16], and liquid membrane extraction [17] presents good prospect with dilute reagents, few unit operations and low energy costs. These processes convert the elements of less value (or whose extraction is not profitable) into a compact and inert residue for landfill. But at present, these methods have few industrial applications, deserving further research for promotion of selenium recovery extraction technology.
Selenium occurs in oxidation states of IV and VI under aqueous conditions. The behavior of selenium in solutions has a close connection with the selenium concentration/potential and pH value which can be represented in the equilibrium predominance diagrams expediently. φ-pH diagram [18] is the important basis for thermodynamics analysis of the process of leaching selenium residue. During selenium extraction from various materials, sulfuric acid solutions are commonly found in processes of reduction precipitation of selenium. The role of sulfate anions in the ionic composition of the solution is usually not considered in the previously reported predominance diagrams. There is, therefore, a need to develop, extend and update the data on new species which come to be recognized to be significant. But at present, very little work on the thermodynamical properties of the Se-H2O system and SO2-Se-H2O system has been published.
According to the thermodynamical data, the behavior of both the Se-H2O and SO2-Se-H2O system in the form of φ-pH diagram is presented by using average heat capacity computation method [19] and through MATLAB programming to provide some useful information for hydrometallurgical process development. On this basis, a new process for producing pure selenium directly from complicated selenium residue was presented and a series of leaching and reducing experiments were conducted.
2 Thermodynamical data and equilibrium equations
According to Eqs. (1)-(3) and aqueous ionic heat capacity parameter (α(T) and β(T)) [19-22], the thermo- dynamical data [23-26] of Se-H2O system (298 K and 373 K) and SO2-H2O system (298 K) were determined. Values of the Gibbs energy of formation ( ), entropy (
), entropy ( ), and aqueous ionic heat capacity
), and aqueous ionic heat capacity 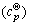 of Se-H2O system at 298 K and 373 K are presented in Table 1. Values of the Gibbs energy of formation (
of Se-H2O system at 298 K and 373 K are presented in Table 1. Values of the Gibbs energy of formation (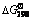 ) of SO2-H2O system at 298 K are given in Table 2.
) of SO2-H2O system at 298 K are given in Table 2.

 (1)
(1)
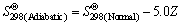 (2)
(2)
 (3)
(3)
On base of the equilibrium principle in hydrometallurgy process, the reaction process can be presented as [18, 27]
aA+mH++Ze=bB+cH2O (4)
According to  =-RTlnK=-ZφF [18, 27], just given the parameters
=-RTlnK=-ZφF [18, 27], just given the parameters  or K, the values of relevant potential φ could be calculated. The balance potential expression could be expressed by Eq. (5) or (6) according to Nernst isotherm equations (Z is aqueous ionic charge):
or K, the values of relevant potential φ could be calculated. The balance potential expression could be expressed by Eq. (5) or (6) according to Nernst isotherm equations (Z is aqueous ionic charge):
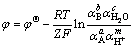 (5)
(5)
 (6)
(6)
Table 1 Thermodynamical data of Se-H2O system at 298 K and 373 K

Table 2 Thermodynamical data of SO2-H2O system at 298 K

Table 3 lists the relationship between relevant potential φ and pH of Se-H2O system at 298 K and 373 K. Simultaneously, the relationship between relevant potential φ and pH of SO2-H2O system at 298 K is presented in Table 4.
Table 3 Relationship between relevant potential φ and pH of Se-H2O system at 298 K and 373 K

Table 4 Relationship between relevant potential φ and pH of SO2-H2O system at 298 K

3 Calculated results and discussion
In all diagrams, broken lines are served to describe boundaries at which the available selenium is equally distributed between the two ionic species which predominate in the contiguous areas.
According to thermodynamical data, the relationship between relevant potential φ and pH of Se-H2O system under 298 K and 373 K in Table 3, the φ-pH diagram of Se-H2O system with activity of 1 of the dissolved selenium species at 298 K and 373 K are shown in Fig. 1 and Fig. 2, respectively.
The diagram can be discussed sequentially in terms of the various selenium species, considering first the behavior of Se(VI), then Se (IV) and Se (0).

Fig. 1 φ-pH diagram of Se-H2O system at 298 K (Activity of dissolved selenium of 1)
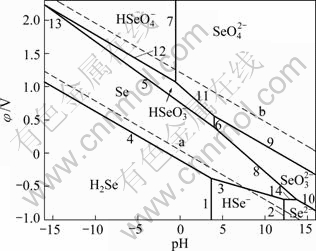
Fig. 2 φ-pH diagram of Se-H2O system at 373 K (Activity of dissolved selenium of 1)
Figure 1 shows that at low pH, hexavalent selenium forms selenic ion  . The isoelectric point is pH 1.65, at which the selenium is changed into complex selenic ion. Hexavalent selenium dissolves in acid to form selenic ion
. The isoelectric point is pH 1.65, at which the selenium is changed into complex selenic ion. Hexavalent selenium dissolves in acid to form selenic ion 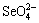 in leaching solution at pH>1.65. For tetravalent selenium, at low pH, the selenic ion in the leaching solution is selenic ion
in leaching solution at pH>1.65. For tetravalent selenium, at low pH, the selenic ion in the leaching solution is selenic ion  , which will be oxidized at about pH 7.26, converting into selenic ion
, which will be oxidized at about pH 7.26, converting into selenic ion  . Selenium dissolves in acid to form selenic ion
. Selenium dissolves in acid to form selenic ion  in leaching solution at pH<7.26. When raising the pH value, selenic ion
in leaching solution at pH<7.26. When raising the pH value, selenic ion  is formed, which is more stable than
is formed, which is more stable than  . Compared to Fig. 1, Fig. 2 shows that at 373 K the isoelectric point is changed to pH=-0.5. Selenic ion
. Compared to Fig. 1, Fig. 2 shows that at 373 K the isoelectric point is changed to pH=-0.5. Selenic ion  dissolves in acid to form selenic ion
dissolves in acid to form selenic ion 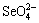 in leaching solution at pH>-0.5. For tetravalent selenium, selenium dissolves in acid to form selenic ion
in leaching solution at pH>-0.5. For tetravalent selenium, selenium dissolves in acid to form selenic ion  in leaching solution at pH<3.97. When raising the pH value, selenic ion
in leaching solution at pH<3.97. When raising the pH value, selenic ion  is formed.
is formed.
In order to further analyze the change of each area, Fig. 3 shows the composition of φ-pH diagram of Se-H2O system at 298 K and 373 K for the purpose of comparison of the variation of these stable regions. It can be found that the speciation of selenium in solution is complex and highly dependent on various selenium species. In the aqueous solution at 298 K and 373 K
(pH≤2), the stable regions of  disappear. That is to say, Se is very hard to be oxidized to
disappear. That is to say, Se is very hard to be oxidized to  (pH≤2), but it can be easily oxidized to
(pH≤2), but it can be easily oxidized to and then
and then  (-5
(-5
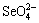 (1.65
(1.65
 ,
, and
and 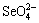 in the
in the
φ-pH diagram of Se-H2O system become gradually large, but the limits of pH in the stable region become gradually small. In general, the stable regions of these areas move towards pH value reducing direction with increasing temperature. In the process of oxidization leaching, Se is first oxidized to  by adding NaClO3 (pH≤2). The oxidization reaction can be expressed by
by adding NaClO3 (pH≤2). The oxidization reaction can be expressed by
 =
=
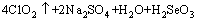 (7)
(7)
 in the oxidization leaching solution is then oxidized to
in the oxidization leaching solution is then oxidized to  or
or 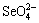 by adding excessive NaClO3, so the oxidization reaction can be expressed by
by adding excessive NaClO3, so the oxidization reaction can be expressed by
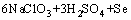 =
=
 (8)
(8)
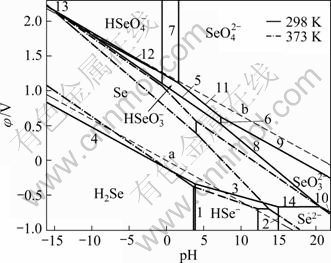
Fig. 3 Composition of φ-pH diagram of Se-H2O system at 298 K and 373 K (Activity of dissolved selenium of 1)
Recovery of selenium from selenium residue in leaching process is dependent on the nature of selenium species that can exist in solution at different potential and pH conditions. Therefore, the φ-pH diagram of Se-H2O system can be used to explain and predict the selenium precipitation.
According to the thermodynamical data present in Table 4, the φ-pH diagram of SO2-H2O at 298 K is shown in Fig. 4. It can be seen that at low pH, hexavalent sulfur forms sulfuric ion  . The isoelectric point is pH 2.18. Hexavalent sulfur dissolves in acid to form sulfuric ion
. The isoelectric point is pH 2.18. Hexavalent sulfur dissolves in acid to form sulfuric ion  in leaching solution at pH>2.18. For tetravalent sulfur, at low pH, the ion in the leaching solution is sulfuric ion
in leaching solution at pH>2.18. For tetravalent sulfur, at low pH, the ion in the leaching solution is sulfuric ion 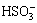 , which will convert into sulfuric ion
, which will convert into sulfuric ion 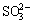 at pH>7.90. SO2 dissolves in acid solution to form sulfuric ion
at pH>7.90. SO2 dissolves in acid solution to form sulfuric ion  in leaching solution at pH>0.68. When raising the pH value (pH>7.90), sulfuric ion
in leaching solution at pH>0.68. When raising the pH value (pH>7.90), sulfuric ion  is formed.
is formed.

Fig. 4 Pourbaix diagram of SO2-H2O system at 298 K (Activity of dissolved sulfur of 1, p(SO2)=105Pa)
The pourbaix diagram of SO2-Se-H2O system at 298 K is represented in Fig. 5 for the purpose of the variation of selenium species in processes of reduction precipitation selenium (pH from -5 to 2). In the acid solution by adding Na2SO3 or NaHSO3, the main reactions are expressed as Eqs. (9) and (10). In essence, the ultimate aim is to produce reducing agent, SO2.

 (9)
(9)

 (10)
(10)
Sulfuric ion 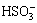 dissolves in acid leaching solution (-5
dissolves in acid leaching solution (-5
2 at pH<0.68. The reducing agent, SO2, in acid solution can firstly reduce selenic ion  (-5
(-5
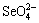 (1.65
(1.65
 in acid leaching
in acid leaching
solution, which will precipitate and convert into elementary substance Se when pH value is less than or equal to 2. According to Fig. 3, Se in the leaching
solution is very hard to be oxidized to  (pH<2), but it can be easily oxidized to be
(pH<2), but it can be easily oxidized to be  and then
and then  (-5
(-5
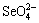 (1.65
(1.65
 by adding NaClO3. The process that reducing agent, SO2, reduces
by adding NaClO3. The process that reducing agent, SO2, reduces  to Se in oxidization leaching solution (pH<2) is thermodynamically feasible. The reduction reaction is expressed by
to Se in oxidization leaching solution (pH<2) is thermodynamically feasible. The reduction reaction is expressed by
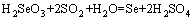 (11)
(11)

Fig. 5 Pourbaix diagram of SO2-Se-H2O system at 298 K
4 Experimental conformation to calculated results
In extra selenium residue of the analysis grade, the mass ratio of selenium residue to sodium chlorate is 2, and the concentration of sulfuric acid is 300 g/L. Then, the residue has been agitated for 3 h with leaching temperature of 80 °C. Finally, the selenium concentrations of the filtered solution and leaching slag have been analyzed. Elemental composition of leaching solution and leaching slag obtained under optimum conditions is given in Table 5.
Table 5 Elemental composition of leaching solution and leaching slag obtained under optimum conditions

In Table 5, it is indicated that after oxidization- leaching under this condition, the leaching rate of selenium could reach 97.76 %. The experimental adding of sodium chlorate to oxidize selenium is thermodynamically feasible. Selenium can be basically leached thoroughly, which explains that the following reaction takes place when the selenium is concentrated in the oxidization-leaching solution. The experimental results conform the calculated results by φ-pH diagram.

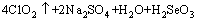 (13)
(13)
Selenium reduction with adding sodium sulphite in oxidization-leaching solution is analyzed under the conditions of acidity of 150 g/L, the sodium sulphite concentration of 35 g/L at the reductive temperature of 23 °C for 120 min. The chemical composition of reduction solution and the grade of selenium powder is presented in Table 6 and Table 7. The XRD of selenium powder is shown in Fig. 6.
Table 6 Chemical composition of reduction solution (mass concentration, g/L)

Table 7 Grade of selenium powder (mass fraction, %)


Fig. 6 XRD pattern of selenium powder
From Table 6 and Table 7, it is indicated that selenium is finally precipitated by adding sodium sulphite in the oxidation leaching solution. The rate of selenium reduction precipitation reaches 99.7%, and the grade of selenium powder reaches up 92.80%. The reducing agent, SO2, reduces  to Se in leaching solution. The experiment results demonstrate the thermodynamic analysis which explains that the following reaction takes place:
to Se in leaching solution. The experiment results demonstrate the thermodynamic analysis which explains that the following reaction takes place:
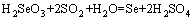 (14)
(14)
Finally, it can be seen from Fig. 6 that the diffraction intensity of selenium powder is not strong, which is mainly formed in amorphous state.
5 Conclusions
1) The φ-pH diagrams of Se-H2O system at 298 K and 373 K show that in the aqueous solution (pH≤2), Se is very hard to be oxidized to be  , but it can be easily oxidized to be
, but it can be easily oxidized to be  and then
and then  (-5
(-5
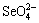 (1.65
(1.65
 ,
,  and
and 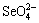 become gradually large, but the limits of pH in the stable region become gradually small. Simultaneously, the φ-pH diagram of SO2-Se-H2O system at 298 K indicates that SO2 can reduce
become gradually large, but the limits of pH in the stable region become gradually small. Simultaneously, the φ-pH diagram of SO2-Se-H2O system at 298 K indicates that SO2 can reduce  to Se in the process of selenium reduction precipitation (pH<2).
to Se in the process of selenium reduction precipitation (pH<2).
2) When the mass ratio of selenium residue to sodium chlorate is 2, the concentration of sulfuric acid is 300 g/L, and the residue is agitated for 3 h at leaching temperature of 80 °C, the leaching rate of selenium could reach 97.76 % in the process of oxidization leaching. This conforms the calculated results by φ-pH diagram. The selenium reduction precipitation in oxidization- leaching solution is analyzed under the conditions of acidity of 150 g/L, the sodium sulphite concentration of 35 g/L at the reductive temperature of 23 °C for 120 min. And this demonstrates the thermodynamic analysis.
References
[1] WHO Working Group. Selenium [J]. Environmental Health Criteria, 1987, 58(1): 306-311.
[2] ZHOU Ling-zhi. Scare metal extraction metallurgy [M]. Beijing: Metallurgical Industry Press, 2008: 281-316. (in Chinese)
[3] ZHOU Ling-zhi. Handbook of scattered metals [M]. Changsha: Central South University of Technology Press, 1993: 372-374. (in Chinese)
[4] GE Qing-hai, CHEN Hou-xing, XIE Ming-hui. Selenium resources application and the studying situation on separation and abstraction technology [J]. Sichuan Nonferrous Metals, 2005, 9(3): 7-15. (in Chinese)
[5] FENG Cai-xia, LIU Jia-jun, LIU Shen. An outline of selenium resources and its exploitation and utilization [J]. Geology and Resources, 2002, 11(3): 152-156. (in Chinese)
[6] HONG Zuo-min. Selenium resource of China: An outline [J]. Geology of Chemical Minerals, 1997, 19(1): 37-42. (in Chinese)
[7] YANG Chang-jiang, ZHANG Xu, LAN De-jun. Present situation and trend of selenium removal technique from copper anode slime [J]. Sichuan Nonferrous Metals, 2005(1): 22-25. (in Chinese)
[8] ZHOU Ben, ZHAO Chen. Plant practice of process optimizing for treating anode slime [J]. Nonferrous Metals, 2003(1): 26-28. (in Chinese)
[9] YIN Shan-ji, LIU Shi-wu, ZHANG De-jie. Practice of increasing selenium recovery rate from copper anode sludge [J]. China Nonferrous Metallurgy, 2008(3): 28-29. (in Chinese)
[10] ZHOU Ling-zhi, CHEN Shao-chun. Scattered metal extraction metallurgy [M]. Beijing: Metallurgical Industry Press, 2008: 285-292. (in Chinese)
[11] CAI Shi-bing. Separation and recovery of selenium and tellurium from high grade waste material [J]. Hydrometallurgy of China, 2008, 27(1): 35-37. (in Chinese)
[12] MANDRINO D, MILUN M, JENKO M. UHV Se evaporation source: Room-temperature deposition on a clean V (110) surface [J]. Institute of Metals and Technology, 2003, 71(1/2): 267-271.
[13] HUANG Zhan-chao, YANG Bin, DAI Yong-nian. Study on vacuum distillation of selenium residue [J]. Yunnan Metallurgy, 2002, 31(6): 27-29. (in Chinese)
[14] TANG Jia-dao, YANG Xiao-ming. Evolution of crude selenium production process in Yunnan copper Co. Ltd [J]. Yunnan Metallurgy, 2008, 37(4): 31-33. (in Chinese)
[15] WEI Zhi-xian, XU Chun-yan. Solvent extraction of Se (IV) from HCl solution [J]. Journal of North China Institute of Technology, 1996, 17(4): 319-322. (in Chinese)
[16] YANG Wen-bin, WANG Jing-fang, WANG Jian-min. Ion exchange from copper anode slime extract pure selenium [J]. Chinese Journal of Rare Metals, 1989, 13(4): 300-303. (in Chinese)
[17] PEI Liang, HAN Fei, WANG Shuang, WANG Li-ming, ZHANG Lei. New progress of application of ionic liquids in extraction and liquid membrane process [J]. Journal of Analytical Science, 2010, 26(3): 347-352. (in Chinese)
[18] MA Rong-jun. Principle on hydrometallurgy [M]. Beijing: Metallurgical Industry Press, 2007: 340-341. (in Chinese)
[19] JIN Zhe-nan, JIANG Kai-xi, WEI Xu-jun, WANG Hai-bei. Potential-pH diagrams of As-S-H2O system at high temperature [J]. Mining and Metals, 1999, 8(4): 45-50. (in Chinese)
[20] YI Xian-wu. An empirical estimation of standard entropy for some complex cations and the E-pH diagrams of As-H2O system at elevated temperature [J]. Journal of Kunming University of Science and Technology, 1982(3): 58-73. (in Chinese)
[21] CRISS C M, COBBLE J W. The thermodynamic properties of high temperature aqueous solutions. IV. Entropies of the ions up to 200 °C and the correspondence principle [J]. Journal of the American Chemical Society, 1964, 86(24): 5385-5390.
[22] ZHONG Zhu-qian, MEI Guang-gui. Application of diagrams of chemical potential in hydrometallurgy and purification of waste water [M]. Changsha: Central South University of Technology Press, 1986: 27-66. (in Chinese)
[23] LIN Chuan-xian, BAI Zheng-hua, ZHANG Zhe-ru. Minerals and related compounds thermodynamics data handbook [M]. Beijing: Science Press, 1985: 31-36. (in Chinese)
[24] LIANG Ying-jiao. Handbook of thermodynamic data of inorganic compounds [M]. Beijing: Metallurgical Industry Press, 2002: 50-89. (in Chinese)
[25] DEAN J A. Lange’s handbook of chemistry [M]. Beijing: Science Press, 2003: 1467-1638.
[26] YANG Xian-wan. Handbook of thermodynamic data in aqueous solutions at high temperature [M]. Beijing: Metallurgical Industry Press, 1983: 523-674. (in Chinese)
[27] FU Cong-yue. Thermodynamic principle and calculation in metallurgical solutions [M]. Beijing: Metallurgical Industry Press, 1989: 154-202. (in Chinese)
(Edited by YANG Bing)
Foundation item: Project(51072233) supported by the National Natural Science Foundation of China
Received date: 2011-09-02; Accepted date: 2011-11-29
Corresponding author: ZHANG Bao, Associate Professor, PhD; Tel: +86-13808415616; Fax: +86-731-88836357; E-mail: csuliqian@csu.edu.cn, csuzb@vip.163.com