Distribution and transformation behaviors of heavy metals during liquefaction process of sewage sludge in ethanol-water mixed solvents
来源期刊:中南大学学报(英文版)2019年第10期
论文作者:黄华军 何小武 潘紫倩 周春飞 赖发英 熊江波 肖晓峰
文章页码:2771 - 2784
Key words:sewage sludge; liquefaction; ethanol-water cosolvent; heavy metals; contamination degree; ecological risk
Abstract: Liquefaction of sewage sludge (SS) in ethanol-water cosolvents is a promising process for the preparation of bio-oil/biochar products. Effect of the combined use of ethanol and water on the distribution/transformation behaviors of heavy metals (HMs) contained in raw SS is a key issue on the safety and cleanness of above liquefaction process, which is explored in this study. The results show that pure ethanol facilitates the migration of HMs into biochar products. Pure water yields lower percentages of HMs in mobile/bioavailable speciation. Compared with sole solvent treatment, ethanol-water cosolvent causes a random/average effect on the distribution/transformation behaviors of HMs. After liquefaction of SS in pure water, the contamination degree of HMs is mitigated from high level (25.8 (contamination factor)) in raw SS to considerable grade (13.4) in biochar and the ecological risk is mitigated from moderate risk (164.5 (risk index)) to low risk (78.8). Liquefaction of SS in pure ethanol makes no difference to the pollution characteristics of HMs. The combined use of ethanol and water presents similar immobilization effects on HMs to pure water treatment. The contamination factor and risk index of HMs in biochars obtained in ethanol-water cosolvent treatment are 13.1-14.6 (considerable grade) and 79.3-101.0 (low risk), respectively. In order to further control the pollution of HMs, it is preferentially suggested to improve the liquefaction process of SS in ethanol-water mixed solvents by introducing conventional lignocellulosic/algal biomass, also known as co-liquefaction treatment.
Cite this article as: PAN Zi-qian, HUANG Hua-jun, ZHOU Chun-fei, LAI Fa-ying, HE Xiao-wu, XIONG Jiang-bo, XIAO Xiao-feng. Distribution and transformation behaviors of heavy metals during liquefaction process of sewage sludge in ethanol-water mixed solvents [J]. Journal of Central South University, 2019, 26(10): 2771-2784. DOI: https://doi.org/10.1007/s11771-019-4212-6.

J. Cent. South Univ. (2019) 26: 2771-2784
DOI: https://doi.org/10.1007/s11771-019-4212-6
PAN Zi-qian(潘紫倩)1, HUANG Hua-jun(黄华军)1, ZHOU Chun-fei(周春飞)2, LAI Fa-ying(赖发英)1,
HE Xiao-wu(何小武)1, XIONG Jiang-bo(熊江波)1, XIAO Xiao-feng(肖晓峰)1
1. School of Land Resources and Environment, Key Laboratory of Agricultural Resource and Ecology in the Poyang Lake Basin of Jiangxi Province, Jiangxi Agricultural University, Nanchang 330045, China;
2. School of Gardening and Landscape Design, Jiangxi Agricultural University, Nanchang 330045, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract: Liquefaction of sewage sludge (SS) in ethanol-water cosolvents is a promising process for the preparation of bio-oil/biochar products. Effect of the combined use of ethanol and water on the distribution/transformation behaviors of heavy metals (HMs) contained in raw SS is a key issue on the safety and cleanness of above liquefaction process, which is explored in this study. The results show that pure ethanol facilitates the migration of HMs into biochar products. Pure water yields lower percentages of HMs in mobile/bioavailable speciation. Compared with sole solvent treatment, ethanol-water cosolvent causes a random/average effect on the distribution/transformation behaviors of HMs. After liquefaction of SS in pure water, the contamination degree of HMs is mitigated from high level (25.8 (contamination factor)) in raw SS to considerable grade (13.4) in biochar and the ecological risk is mitigated from moderate risk (164.5 (risk index)) to low risk (78.8). Liquefaction of SS in pure ethanol makes no difference to the pollution characteristics of HMs. The combined use of ethanol and water presents similar immobilization effects on HMs to pure water treatment. The contamination factor and risk index of HMs in biochars obtained in ethanol-water cosolvent treatment are 13.1-14.6 (considerable grade) and 79.3-101.0 (low risk), respectively. In order to further control the pollution of HMs, it is preferentially suggested to improve the liquefaction process of SS in ethanol-water mixed solvents by introducing conventional lignocellulosic/algal biomass, also known as co-liquefaction treatment.
Key words: sewage sludge; liquefaction; ethanol-water cosolvent; heavy metals; contamination degree; ecological risk
Cite this article as: PAN Zi-qian, HUANG Hua-jun, ZHOU Chun-fei, LAI Fa-ying, HE Xiao-wu, XIONG Jiang-bo, XIAO Xiao-feng. Distribution and transformation behaviors of heavy metals during liquefaction process of sewage sludge in ethanol-water mixed solvents [J]. Journal of Central South University, 2019, 26(10): 2771-2784. DOI: https://doi.org/10.1007/s11771-019-4212-6.
1 Introduction
Sewage sludge (SS), an inevitable by-product of municipal wastewater treatment, has become a key issue in many countries due to its increasing volume and the environmental impacts associated with its disposal [1, 2]. It is a critical biologically active mixture of water, organic matter (derived from human wastes, food wastes, etc.), dead and alive microorganisms (including pathogens), and inorganic and organic toxic contaminants (e.g., metallic trace elements, PAHs). The desired new/upgraded technologies should allow for a full recycling of organic matter and reduction of the potential risk associated with the presence of pollutants [3]. Conventional methods of SS management, for example, landfilling, incineration and agricultural application, are no longer sustainable due to the stringent regulations, the shortage of land space and the rising environmental and health concerns on the presence of harmful substances [4-6].
In recent years, thermochemical liquefaction of SS with proper solvents for bio-oil and biochar products is considered to be a promising technology, which has attracted a lot of attention [5, 7]. Considering that SS usually has high content of water (75%-80%, after mechanical dehydration) [8, 9], water is the most widely used solvent in the liquefaction of SS [10-15]. To obtain higher yield/caloric value of water-insoluble bio-oil and relatively mild reaction conditions, organic solvents, such as acetone, methanol and ethanol, were tested in the liquefaction of SS [16-20]. However, the use of organic solvent is also faced with some problems, such as high cost of liquefaction solvent, high energy consumption of SS drying and environmental pollution concerns. Recent studies indicate that organic solvent-water cosolvents may be more proper for the liquefaction of SS, which combines the advantages of water and organic solvent [21-23].
Heavy metals (HMs) are one of the most common inorganic pollutants in SS. Thus, the distribution and transformation behaviors of HMs during the liquefaction of SS have become a research hotspot [7, 24]. HUANG et al [25] and YUAN et al [26] first reported the change situations of HMs’ concentration, chemical speciation and environmental risk during the liquefaction of SS in acetone. ZHAI et al [27], CHEN et al [28] and LENG et al [29] focused on the effects of the addition of catalyst or lignocellulosic biomass during the liquefaction of SS in water or ethanol on the distribution and transformation behavior of HMs. LENG et al [30] and YUAN et al [31] compared the concentration, chemical speciation and environmental risk of HMs in bio-oil and biochar products derived from the liquefaction of SS in acetone and ethanol. SHAO et al [32] explored the variation characteristics of HMs during the liquefaction of SS with methanol as solvent.
In a previous work of the authors [21], the liquefaction of SS in ethanol-water co-solvents for bio-oil and biochar products was studied. It has been reported that there existed synergetic effects between ethanol and water on the liquefaction of SS. In special, higher yield/quality of bio-oil products were obtained at mild reaction conditions. In addition, the biochar products possessed similar specific area and pore volume to SS pyrolysis biochar. Whether there exists some interaction between ethanol and water or not, influencing the variation characteristics of HMs during the liquefaction of SS, is a key topic on the safety and cleanness of above liquefaction process, which is still unsolved. The primary object of this study is to fill this gap. In this study, the distribution and transformation behaviors of HMs during the liquefaction of SS in ethanol-water mixed solvents were explored. For comparative purpose, the change situations of HMs in the liquefaction of SS with pure water or pure ethanol as solvent were also investigated in this study.
2 Materials and methods
2.1 Materials
Raw SS materials were obtained from a wastewater treatment plant located in Nanchang City, Jiangxi Province (China). The main treatment technology of wastewater and sludge are oxidation ditch and air flotation thickening/mechanical dewatering, respectively. Four bio-oil samples, marked as PW-O, PE-O, E-W-O and E-W-C-O, were collected from the liquefaction of SS in pure water, pure ethanol and isometric ethanol-water (with or without NaOH), respectively. The corresponding biochar samples were labeled as PW-C, PE-C, E-W-C and E-W-C-C, respectively. Other optimized liquefaction conditions, such as reaction temperature, reaction time and solid-liquid ratio, were 220 °C, 30 min and 0.1 g/mL, respectively. The specific liquefaction process is detailedly described in a previous work [21]. The pretreatment processes and basic physicochemical characteristics of SS and biochar/bio-oil samples can also refer to the above-mentioned work. All chemicals involved in the experiments mentioned later, including acetic acid, hydroxylammonium chloride and ammonium acetate, etc., are all of analytical reagent grade.
2.2 Analysis of HMs’ total content
Mixed-acid digestion method was adopted to determine the total content of HMs in raw SS and bio-oil/biochar products. In special, firstly weighing 0.2 g of each sample, then successively adding 5 mL HNO3, 5 mL HClO4 and 3 mL H2O2 (30%) and lastly digesting the mixtures on an electric hot plate (EH35B, LabTech, China) till nearly dry (about two drops). After cooling down, the residue was firstly dissolved with 5% HNO3, then filtered with 0.45 μm filter membrane and transferred into a 50 mL of volumetric flask. Finally, the volume was fixed to the mark line [33].
2.3 Analysis of HMs’ chemical speciation
The BCR sequential extraction procedure was applied to analyzing the chemical speciation of HMs in SS and biochar samples, containing four following steps [34]: Step one (F1, exchangeable metal and carbonate-associated fractions): 0.5 g of each sample was mixed with 20 mL of acetic acid (0.1 mol/L) in a 50 mL of centrifuge tube, and then shaken at room temperature for 16 h. Then the intermixtures were separated by centrifugation at 4000 r/min for 20 min. Subsequently, the supernatant was collected and filtered through a 0.45 μm filter membrane. The solid residues were preserved for subsequent extractions. Step two (F2, reducible fraction): the residues from step one were mixed with 20 mL of hydroxylammonium chloride (0.1 mol/L, pH=2) and then shaken at room temperature for 16 h. The subsequent solid-liquid separation and supernatant purification were the same as that described in step one. Step three (F3, oxidizable fraction): The residues from step two were firstly dissolved with 5 mL of 30% H2O2 and then digested at room temperature for 1 h with intermittent oscillation. Another 5 mL of 30% H2O2 was added, and then the mixtures were digested and evaporated at 85 °C (water bath) for 1 h until a small volume (1-2 mL) of mixtures were remained. Afterwards, 25 mL of ammonium acetate (1.0 mol/L, pH=2) was added and then the mixtures were shaken at room temperature for 16 h. The separation and purification also referred to the procedure described in step one. Step four (F4, residual fraction): The residues from step three were digested according to the method mentioned in Section 2.2.
2.4 Analysis of HMs’ leachable concentration
The leaching characteristics of HMs in SS and biochars were assessed by a toxicity characteristic leaching procedure (TCLP). In special, 1.0 g of each sample was firstly mixed with 20 mL glacial acetic acid solution (pH=2.8) and then shaken at 120 r/min for 20 h. Afterwards, the intermixtures were centrifugated at 4000 r/min for 20 min and the liquid phase was collected and filtered through a 0.45 μm filter membrane [33].
The concentrations of six HMs (Zn, Cu, Pb, Cd, Cr and Ni) in each digestion/extraction solution were determined by an atomic absorption spectrometer (PinAAcle 900T, Perkin Elmer, USA).
2.5 Assessment methods of contamination degree and ecological risk
Assessment methods, applied to evaluating the effects of hydrothermal treatment on the contamination level/risk of HMs in SS, have been systematically summarized and analyzed in a review literature [24]. There are usually two distinct evaluation methods, i.e., the total content indices and the speciation indices. The total content indices are calculated on the basis of the total content of HMs. And the speciation indices are developed in the light of the chemical speciation of HMs [35]. In this study, two speciation indices were adopted as follows:
Global contamination factor (GCF) was used to assess the contamination level of multi-metal [35, 36].
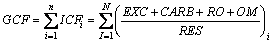 (1)
(1)
where individual contamination factor (ICFi) is the contamination degree of single-metal; EXC, CARB, RO, OM and RES are the percentages of exchangeable fraction, carbonate fraction, reducible oxide fraction, organic matter fraction and residual fraction, respectively.
Global risk index (GRI) was used to assess the ecological risk of multi-metal [35].
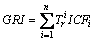 (2)
(2)
where 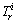 is the toxic factor of metal i. The values for each metal are in the order of
is the toxic factor of metal i. The values for each metal are in the order of 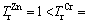
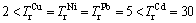
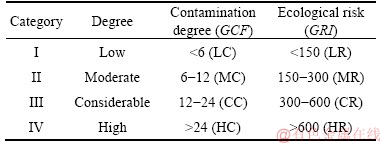
The contamination level and ecological risk of HMs were both divided into four grades according to GRI and GCF, as shown in Table 1.
Table 1 Categories of heavy metals’ contamination degree and ecological risk [24]
2.6 Definition
The percentages of HMs distributed in liquefaction products are calculated from the total concentrations of HMs in biochar/bio-oil and their corresponding yields (Eq. (3) and Eq. (4)).
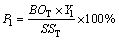 (3)
(3)
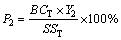 (4)
(4)
where P1 and P2 are the proportions of heavy metals distributed in bio-oils and biochars, respectively, %; BOT, BCT, and SST are the total contents of HMs in bio-oils, biochars and SS, respectively, mg/kg; Y1 and Y2 are the yields of bio-oils and biochars, respectively, %, which can refer to a previous work of the authors [21].
An index, namely, “recovery rate”, is calculated to check on the results of BCR sequential extraction procedure, which is defined as the percentage of the sum of the four fractions to the total concentrations of heavy metals obtained from mixed-acid digestion method. The detailed calculations are expressed as follows:
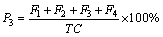 (5)
(5)
where P3 is recovery rate of heavy metals (%); TC is total concentration of heavy metals (mg/kg); F1, F2, F3, F4 are concentrations of heavy metals extracted in each fraction.
The leaching rates (P4) can be used to reflect the proportion of HMs in the leachable state (Eq. (6)).
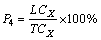 (6)
(6)
where X is one kind of heavy metal; LCX is the leaching content of HMs, mg/kg; TCX is the total content of HMs, mg/kg.
3 Results and discussion
3.1 Distribution characteristics of HMs
Considering that liquefaction experiments are always conducted at relatively low temperature (220 °C in this study), it is assumed that few HMs will be transferred to the gas phase products [37]. Thus, the content of HMs in gaseous products is not analyzed. During the liquefaction process, the unstable or loosely absorbed HMs in raw SS will be firstly released/dissolved, and then redistributed into the liquid/solid phase products (bio-oil and bio-char) [38]. The total contents of HMs in raw SS and its liquefaction products are listed in Table 2. Figure 1 presents the distribution characteristics of HMs in bio-oil/biochar derived from the liquefaction of SS.
As shown in Figure 1, after liquefaction, HMs contained in raw SS will mainly distribute into the solid phase product (biochars). In special, Cu, Pb and Cd are not detected in bio-oil products. In other words, the overwhelming majority of Cu, Pb and Cd are concentrated in biochars. The percentages of Cu, Pb and Cd distributed in biochars are all over 90%, except the biochar, produced from the liquefaction of SS in ethanol-water cosolvent with NaOH as catalyst, contains 86.7% of Cu. As regards to Ni, Zn and Cr, a certain amount of them are transferred into bio-oils. About 5% of Zn and Cr are detected in bio-oil, while the percentages of Ni distributed in bio-oils are ranked in the range of 6%-19%. In biochars, the percentages of Cr are around 90%, while Zn presents a relatively low percentage, varying around 70%. The percentages of Ni transferred into biochars vary in a wide range of 76%-94%.
In 2009, China issued a national standard, namely, ‘Disposal of Sludge from Municipal Wastewater Treatment Plant–Quality of Sludge Used in Land Improvement’ [39]. In this national standard, the threshold values of HMs in disposed SS are specified, which are also shown in Table 2. Before liquefaction, the total contents of HMs in raw SS, except Ni and Cu, are all under the threshold values of acid soils (pH<6.5). The content Cu slightly exceeds the safe level of acid soils but still under that of neutral and alkaline soils (pH≥6.5). Worryingly, the concentration of Ni is very high, significantly exceeding the standard value of neutral and alkaline soils. After liquefaction, the total concentrations of HMs in biochars obviously increase. The total contents of Zn and Pb are still under the prescribed value of acid soils. And the total contents of Cu, Cd and Cr also do not exceed the specified value of neutral and alkaline soils. The content of Ni, to no one’s surprise, does not meet the requirement. There are two rational reasons for explaining the high contents of HMs in biochars: 1) the HMs contained in raw SS are difficult to volatilize and convert during liquefaction process and mainly remain in solid products; 2) most of organic matters in raw SS are decomposed and converted into liquid products, indirectly increasing the content of HMs in biochar [26, 37].
Table 2 Total concentrations of heavy metals in liquefaction products
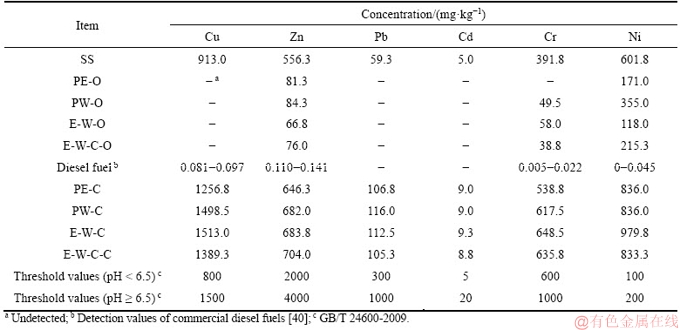
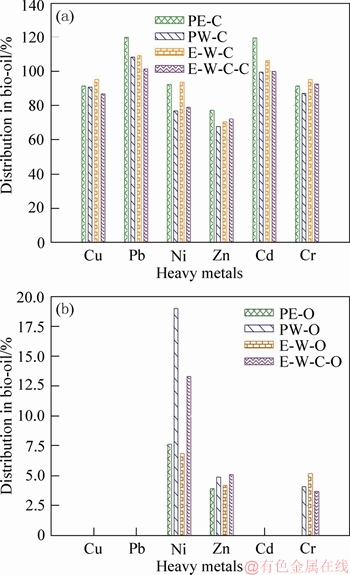
Figure 1 Distribution behaviors of heavy metals
The contents of Zn, Cr and Ni in bio-oil vary in the ranges of 66-85 mg/kg, 38-58 mg/kg and 118-355 mg/kg, respectively. LIM et al [40] detected the contents of HMs in commercial diesel fuels, which are cited in this study and listed in Table 2. Quite evidently, the bio-oil products contain undesirably high contents of Zn, Cr and Ni compared to regular diesel fuels. If the bio-oil products are directly utilized as fuel oil, it is bound to lead to HMs pollution. Therefore, the bio-oil products should be further upgraded to reduce the content of HMs.
Interestingly, compared with pure water, the use of pure ethanol as liquefaction solvent yields higher percentages of HMs in biochars and lower proportions of HMs in bio-oils. As clearly shown in Figure 1, the percentages of all six HMs (Cu, Pb, Ni, Zn, Cd and Cr) in biochars obtained from the liquefaction of SS in pure ethanol are relatively higher. On the contrary, the bio-oils obtained from the liquefaction of SS in pure water possess higher percentages of Ni, Zn and Cr. In other words, pure ethanol may facilitate the migration of heavy metals into biochar products. The use of ethanol-water mixed solvents does not present definite influences on the distribution behaviors of HMs compared with the cases of sole pure solvent. In special, the use of ethanol-water mixed solvents results in higher percentages of Cu, Ni and Cr in biochars. Correspondingly, lower percentage of Ni is migrated into bio-oil. The percentages of Pb, Zn and Cd in biochars fall somewhere in the middle, compared to pure water/ethanol treatments. Similar trend is observed for the percentage of Zn in bio-oil. On the whole, the addition of NaOH weakens the migration of HMs into biochars with elevating the percentage of HMs in bio-oil, except for Cr.
3.2 Transformation characteristics of HMs’ chemical speciation
The bioavailability and eco-toxicity of HMs mainly depend on their specific chemical forms and their binding state (precipitated with primary or secondary minerals, complexed by organic ligands, and so on) [34, 41]. Therefore, the transformation characteristics of HMs during the liquefaction process of SS are further explored by comparing the chemical speciation of HMs in biochars and raw SS. The specific relationships among the chemical speciation, eco-toxicity and bioavailability of HMs have been established by researchers: 1) acid soluble/exchangeable fraction (F1) and reducible fraction (F2) belong to direct effect fractions and have direct toxicity; 2) oxidizable fraction (F3) is classed as potential effect fraction and has potential toxicity; 3) residual fraction (F4) is geared to stable fraction and has no toxicity [24]. The concentrations and percentages of each speciation of HMs in biochars and raw SS are shown in Table 3 and Figure 2, respectively.
When pure ethanol is adopted as liquefaction solvent, the immobilization effects of HMs are insignificant. Although the percentages of Cu and Ni bound to direct effect fractions (F1 and F2) are decreased, the proportions of stable fraction (F4) are little changed. Accordingly, obvious increase in the percentage of potential effect fraction (F3) is observed. As for Cd, Cr and Zn, the proportions of F1 and F2 present no obvious variation. But the scales of F3 increase slightly, instead the percentages of F4 go down accordingly. In the case of Pb, the percentage of F4 is further increased with the decrease of F3.
Table 3 Contents of each fraction of heavy metals in biochar and raw SS (mg/kg)
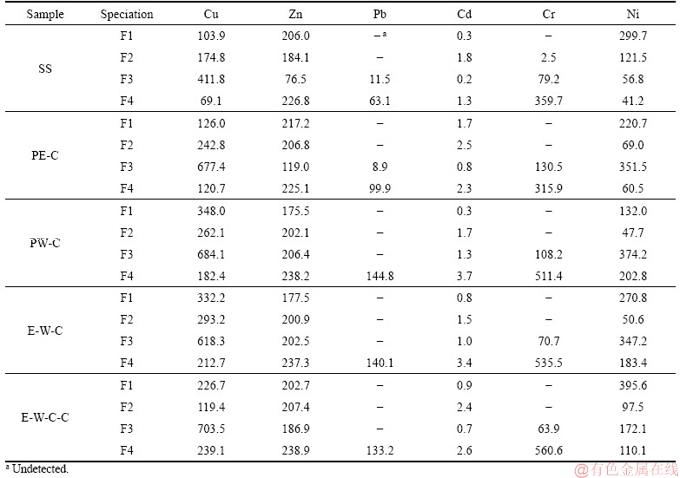
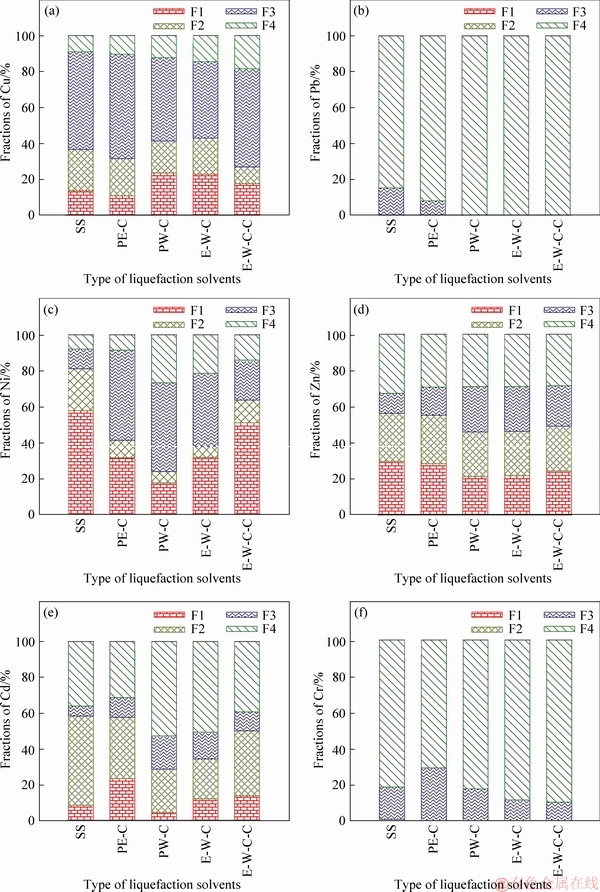
Figure 2 Transformation behaviors of heavy metals (Biochars were produced at 220 °C for 30 min with a solid-liquid ratio of 0.1 g/mL)
Generally, the use of pure water has better immobilization effects of HMs compared with pure ethanol. The ratios of Cd, Ni and Zn distributed in direct effect fractions (F1 and F2) are obviously declined. Correspondingly, the proportions of potential effect/stable fractions (F3 and F4) present significant increase. As regards to Pb, only residual fraction is detected. There is no obvious change in the chemical speciation of Cr. Surprisingly perhaps, although the percentage of Cu associated with stable fraction (F4) somewhat rises, the amounts of F1 and F2 also slightly go up with the decline of potential effect fraction (F3).
The joint use of ethanol and water presents random/average effects on the immobilization effects of HMs. Ethanol-water cosolvents exhibit similar immobilization effects on Zn and Pb, compared to pure water treatment. As regards to Cd and Ni, the immobilization effects of ethanol-water mixed solvents are worse than pure water but better than pure ethanol. In the case of Cr and Cu, the use of ethanol-water cosolvents further improves the amounts of stable fraction (F4), indicating better immobilization effects than pure water. The addition of NaOH in the liquefaction of SS with ethanol-water cosolvents as reaction medium enhances the immobilization of Cu. But, a just opposed trend is observed for Cd and Ni. As for Pb, Zn and Cr, no obvious variation is imparted by the addition of NaOH.
Figure 3 presents the recovery rates of heavy metals extracted by BCR method. On the whole, the sum of the four fractions is in good agreement with the total concentration of heavy metals with satisfactory recoveries (72.0%-126.6%), similar to those reported in literature (71.6%-123.9%) [26, 34,42, 43]. It suggests that BCR sequential extraction method used for analyzing the chemical speciation of Cu, Zn, Pb, Cd, Cr and Ni in SS and biochars is exact and reliable.
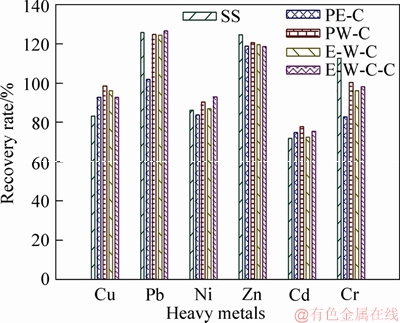
Figure 3 Recovery rate of heavy metals extracted by BCR methods
3.3 Leaching characteristics of HMs
The leaching characteristics of HMs can be used to determine the mobility of HMs [32]. Table 4 summarizes the concentrations of leachable HMs in raw SS and biochar products. Pb and Cr are not detected in the leachate extracted from raw SS and all biochars. The contents of leachable Cd in raw SS and biochars are all under the permissible value (1.0 mg/kg). As for Zn and Ni, the leachable amounts are both higher than threshold values. Compared to raw SS, the concentrations of leachable Zn in biochars change little, while the contents of Ni are obviously declined. In the case of Cu, the leachable contents are unexpectedly increased. On the whole, the liquefaction process does not obviously reduce the leaching risk of heavy metals (except Ni) to the environment. The main reason might be ascribed to the relatively low reaction temperature adopted in the liquefaction of SS (220 °C in this study). SHI et al [44] studied the immobilization effects of HMs in hydrothermal carbonization of SS and reported that when the treatment temperatures were set at 170 °C and 200 °C, the concentrations of some HMs in the leachate of treated SS were also increased compared to raw SS. And when the treatment temperature was elevated to 280 °C, the leachable contents were significantly decreased for all HMs.
The leaching rates (P4) of above six HMs are depicted in Figure 4. Comparing Figure 2 and Figure 4, it can be found that the variation trends of leaching rates show positive correction with that of the percentages of acid soluble/exchangeable fraction (F1). The correlation coefficients (R2) are beyond 0.9 except for Cd (Figure 5). In other words, the HMs extracted in the leachate are mainly originated from the acid soluble/exchangeable fraction of HMs. Obviously, the liquefaction process involved in this study does not effectively reduce the contents of acid soluble/exchangeable HMs (Figure 2 and Table 3), and thus relatively high leachable concentration/ratio of Cu, Cd, Ni and Zn are still observed in biochar products (Figure 3 and Table 4).
Table 4 Leachable metal concentrations based on TCLP test
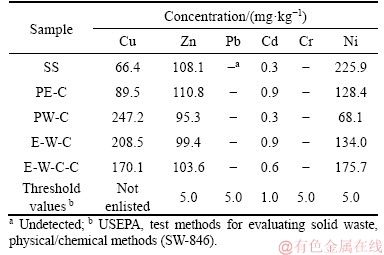
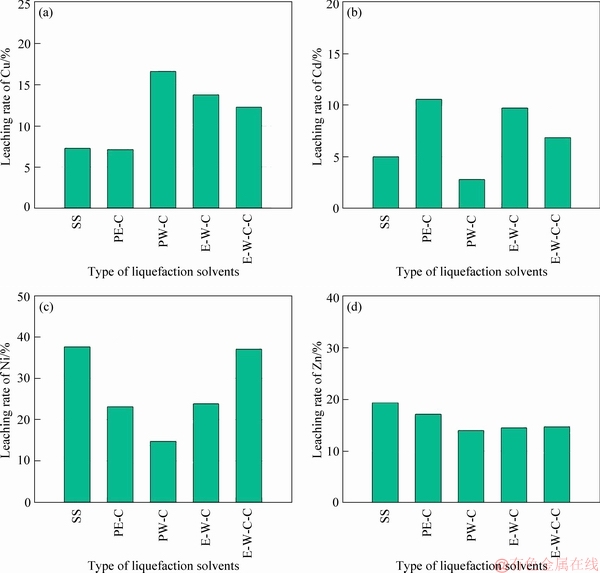
Figure 4 Leachable behaviors of heavy metals
3.4 Contamination degree and ecological risk of HMs
It is a key issue for determining the cleanness of SS liquefaction technology that whether the contamination degree and ecological risk of HMs in SS can be mitigated after liquefaction or not. Thus, in this study, the variation situations of HMs’ contamination degree and ecological risk are quantificationally assessed according to GCF and GRI methods. The corresponding evaluation results are listed in Table 5 and Figure 6. Before liquefaction, the GCF and GRI of HMs in raw SS are as high as 25.8 and 164.5, corresponding to high contamination degree and moderate ecological risk, respectively. In other words, environmental problems caused by HMs will be produced, if SS is directly discharged to the environments without any pretreatment.
The biochar obtained from the liquefaction of SS in pure ethanol presents similar GCF and GRI values to raw SS, which indicates that liquefaction of SS in pure ethanol can not availably mitigate the pollution of HMs. In the case of pure water as liquefaction solvent, the GCF and GRI values of HMs are significantly decreased. In special, the contamination degree of HMs is decreased from high level to considerable grade and the ecological risk is mitigated from moderate risk to low risk. The use of ethanol-water cosolvent in liquefaction of SS does not further mitigate the contamination degree and ecological risk of HMs, close to pure water treatment. These results consisted with the immobilization effects of pure water, pure ethanol and ethanol-water cosolvents on HMs, as discussed in Section 3.2. In addition, the application of NaOH in the liquefaction of SS with ethanol-water mixed solvents as reaction medium does not impart substantial changes on the pollution situation of HMs.
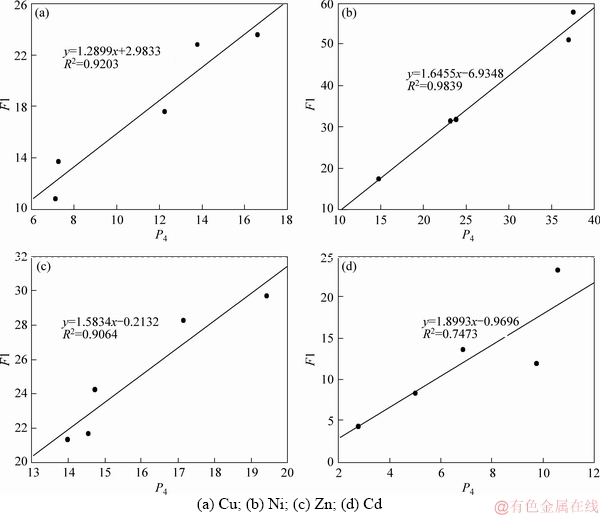
Figure 5 Correlation analysis between P4 and F1:
Table 5 Calculation process of heavy metals’ contamination degree and ecological risk
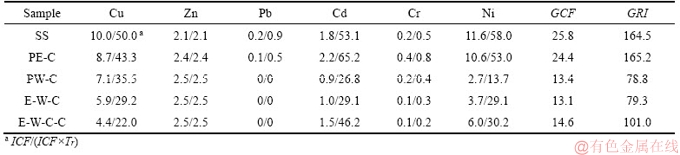
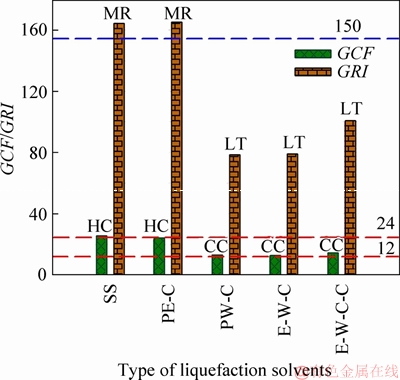
Figure 6 Contamination degree and ecological risk of heavy metals
3.5 Future research suggestions
It has been reported that the use of ethanol-water cosolvent in the liquefaction of SS presents synergetic effects, which makes reaction condition mild (optimized reaction temperature only at 220 °C) and results in higher yield and quality of bio-oil products. Meanwhile, the biochar products obtained in ethanol-water treatments also possess high potential in the fields of land application and adsorption materials [21]. However, in this follow-up study, it is found that the joint use of ethanol and water presents random/average influences on the distribution and transformation behaviors of HMs during the liquefaction process of SS, compared to pure water/ethanol treatments. On the whole, the pollution situation of HMs contained in SS is not significantly mitigated after the liquefaction process of SS in ethanol-water mixed solvents. In special, the content of HMs in bio-oil is undesirably high. Although the ecological risk of HMs in biochar is lowered to low risk level, the pollution degree is still up to considerable contamination level.
The content of ash in SS is generally higher than conventional lignocellulosic and algal biomass, resulting higher yield of solid phase product (biochar, byproduct). Thus, the proper utilization of above byproduct is especially of importance. From the view of HMs’ pollution situation in liquefaction products, the liquefaction of SS in ethanol-water should be further improved. For example, conventional lignocellulosic and algal biomass, containing low content of HMs, can be introduced into the liquefaction of SS [16, 29, 45]. In addition, liquefaction of SS pretreated by dementalization procedures is also a reliable route [17]. The passivation or removal of HMs in liquefaction products before application might be people’s last and unwilling choice.
4 Conclusions
Liquefaction of SS in pure ethanol results in higher percentages of HMs in biochars. The pollution situation of HMs changes little in pure ethanol treatment. When pure water is used as liquefaction solvent, the contamination degree of HMs is decreased from high level to considerable grade and the ecological risk is mitigated from moderate risk to low risk. Ethanol-water cosolvents present random/average effects on the distribution and transformation behaviors of HMs, resulting similar passivation effects to pure water treatment. To improve the cleanness of SS liquefaction in ethanol-water cosolvents, introduction of lignocellulosic/algal biomasses or dementalization pretreatment of SS is suggested.
References
[1] SYED-HASSAN S S A, WANG Yi, HU Song, SU Sheng, XIANG Jun. Thermochemical processing of sewage sludge to energy and fuel: Fundamentals, challenges and considerations [J]. Renewable and Sustainable Energy Reviews, 2017, 80: 888-913. DOI: https://doi.org/10.1016/ j.rser.2017.05.262.
[2] REN Miao-miao, YUAN Xing-zhong, ZHU Yi, HUANG Hua-jun, ZENG Guang-ming, LI Hui, CHEN Ming, WANG Hou, CHEN Chang-ya, LIN Ning-bo. Effect of different surfactants on removal efficiency of heavy metals in sewage sludge treated by a novel method combining bio-acidification with Fenton oxidation [J]. Journal of Central South University, 2014, 21(12): 4623-4629. DOI: 10.1007/s11771-014-2469-3.
[3] KACPRZAK M, NECZAJ E, FIJALKOWSKI K, GROBELAK A, GROSSER A, WORWAG M, RORAT A, BRATTEBO H, ALMAS A, SINGH B R. Sewage sludge disposal strategies for sustainable development [J]. Environmental Research, 2017, 156: 39-46. DOI: https://doi.org/ 10.1016/j.envres.2017.03.010
[4] QIAN Li-li, WANG Shu-zhong, XU Dong-hai, GUO Yang, TANG Xing-ying, WANG Lai-sheng. Treatment of municipal sewage sludge in supercritical water: A review [J]. Water Research, 2016, 89: 118-131. DOI: https://doi.org/10.1016/ j.watres.2015.11.047
[5] MULCHANDANI A, WESTERHOFF P. Recovery opportunities for metals and energy from sewage sludges [J]. Bioresource Technology, 2016, 215: 215-226. DOI: https://doi.org/10.1016/j.biortech.2016.03.075.
[6] OUYANG Jian-xin, SHI Zhou, ZHONG Hua, LIU Wei, CHAI Qi, YUAN Xing-zhong. Static aerobic composting of municipal sewage sludge with forced ventilation: Using matured compost as bulking conditioner [J]. Journal of Central South University, 2014, 21(1): 303-309. DOI: 10.1007/s11771-014-1941-4.
[7] HUANG Hua-jun, YUAN Xing-zhong. Recent progress in the direct liquefaction of typical biomass [J]. Progress in Energy and Combustion Science, 2015, 49: 59-80. DOI: https://doi.org/10.1016/j.pecs.2015.01.003.
[8] LIANG Jia-lin, HUANG Shao-song, DAI Yong-kang, LI Lei, SUN Shui-yu. Dewaterability of five sewage sludges in Guangzhou conditioned with Fenton's reagent/lime and pilot-scale experiments using ultrahigh pressure filtration system [J]. Water Research, 2015, 84: 243-254. DOI: https:// doi.org/10.1016/j.watres.2015.07.041.
[9] MO Ru-song, HUANG Shao-song, DAI Wen-can, LIANG Jia-lin, SUN Shui-yu. A rapid Fenton treatment technique for sewage sludge dewatering [J]. Chemical Engineering Journal, 2015, 269: 391-398. DOI: https://doi.org/10.1016/ j.cej.2015.02. 001.
[10] BILLER P, JOHANNSEN I, DOS-PASSOS J S, OTTOSEN L D M. Primary sewage sludge filtration using biomass filter aids and subsequent hydrothermal co-liquefaction [J]. Water Research, 2018. 130: 58-68. DOI: https://doi.org/10.1016/ j.watres.2017.11.048
[11] MA Wen-chao, DU Gui-yue, LI Jian, FANG Yuan-hao, HOU Li-an, CHEN Guan-yi, MA De-gang. Supercritical water pyrolysis of sewage sludge [J]. Waste Management, 2017, 59: 371-378. DOI: https://doi.org/10.1016/j.wasman.2016. 10.053.
[12] QIAN Li-li, WANG Shu-zhong, SAVAGE P E. Hydrothermal liquefaction of sewage sludge under isothermal and fast conditions [J]. Bioresource Technology, 2017, 232: 27-34. DOI: https://doi.org/10.1016/j.biortech. 2017. 02.017.
[13] MALINS K, KAMPARS V, BRINKS J, NEIBOLTE I, MURNIEKS R, KAMPARE R. Bio-oil from thermo- chemical hydro-liquefaction of wet sewage sludge [J]. Bioresource Technology, 2015, 187: 23-29. DOI: https://doi. org/10.1016/j.biortech.2015.03.093.
[14] WANG Yan, CHEN Guan-yi, LI Yan-bin, YAN Bei-bei, PAN Dong-hui. Experimental study of the bio-oil production from sewage sludge by supercritical conversion process [J]. Waste Management, 2013, 33(11): 2408-2415. DOI: https://doi.org/ 10.1016/j.wasman.2013.05.021.
[15] VARDON D R, SHARMA B K, SCOTT J, YU G, WANG Z C, SCHIDEMAN L, ZHANG Y H, STRATHMANN T J. Chemical properties of biocrude oil from the hydrothermal liquefaction of Spirulina algae, swine manure, and digested anaerobic sludge [J]. Bioresource Technology, 2011, 102(17): 8295-8303. DOI: https://doi.org/10.1016/j.biortech. 2011.06.041.
[16] LENG Li-jian, LI Jun, YUAN Xing-zhong, LI Jing-jing, HAN Pei, HONG Yu-chun, WEI Feng, ZHOU Wen-guang. Beneficial synergistic effect on bio-oil production from co-liquefaction of sewage sludge and lignocellulosic biomass [J]. Bioresource Technology, 2018, 251: 49-56. DOI: https:// doi.org/10.1016/j.biortech.2017.12.018.
[17] LENG Li-jian, YUAN Xing-zhong, SHAO Jian-guang, HUANG Hua-jun, WANG Hou, LI Hui, CHEN Xiao-hong, ZENG Guang-ming. Study on demetalization of sewage sludge by sequential extraction before liquefaction for the production of cleaner bio-oil and bio-char [J]. Bioresource Technology, 2016, 200: 320-327. DOI: https://doi.org/ 10.1016/j.biortech.2015.10.040.
[18] LI Hui, YUAN Xing-zhong, ZENG Guang-ming, HUANG Dan-lian, HUANG Hua-jun, TONG Jing-yi, YOU Qiao, ZHANG Jia-chao, ZHOU Ming. The formation of bio-oil from sludge by deoxy-liquefaction in supercritical ethanol [J]. Bioresource Technology, 2010, 101(8): 2860-6. DOI: https://doi.org/10.1016/j.biortech.2009.10.084.
[19] HUANG Hua-jun, YUAN Xing-zhong, LI Bao-tong, XIAO Yuan-dong, ZENG Guang-ming. Thermochemical liquefaction characteristics of sewage sludge in different organic solvents [J]. Journal of Analytical and Applied Pyrolysis, 2014, 109: 176-184. DOI: https://doi.org/10.1016/ j.jaap.2014.06.015.
[20] HUANG Hua-jun, YUAN Xing-zhong, ZHU Hui-na, LI Hui, LIU Yan, WANG Xue-li, ZENG Guang-ming. Comparative studies of thermochemical liquefaction characteristics of microalgae, lignocellulosic biomass and sewage sludge [J]. Energy, 2013, 56: 52-60. DOI: https://doi.org/10.1016/ j.energy. 2013.04.065.
[21] LAI Fa-yin, CHANG Yan-chao, HUANG Hua-jun, WU Guo-qiang, XIONG Jiang-bo, PAN Zhi-qian, ZHOU Chun-fei. Liquefaction of sewage sludge in ethanol-water mixed solvents for bio-oil and biochar products [J]. Energy, 2018, 148: 629-641. DOI: https://doi.org/10.1016/j.energy. 2018.01.186.
[22] WANG Wen-jia, YU Qi, MENG Han, HAN Wei, LI Jie, ZHANG Jing-lai. Catalytic liquefaction of municipal sewage sludge over transition metal catalysts in ethanol-water co-solvent [J]. Bioresource Technology, 2018, 249: 361-367. DOI: https://doi.org/10.1016/j.biortech.2017.09.205.
[23] PRAJITNO H, PARK J, RYU C, PARK H Y, LIM H S, KIM J. Effects of solvent participation and controlled product separation on biomass liquefaction: A case study of sewage sludge [J]. Applied Energy, 2018, 218: 402-416. DOI: https:// doi.org/10.1016/j.apenergy.2018.03.008.
[24] HUANG Hua-jun, YUAN Xing-zhong. The migration and transformation behaviors of heavy metals during the hydrothermal treatment of sewage sludge [J]. Bioresource Technology, 2016, 200: 991-998. DOI: https://doi.org/10.1016/ j.biortech.2015.10.099.
[25] HUANG Hua-jun, YUAN Xing-zhong, ZENG Guang-ming, ZHU Hui-na, LI Hui, LIU Zhi-feng, JIANG Hong-wei, LENG Li-jian, BI Wen-kai. Quantitative evaluation of heavy metals’ pollution hazards in liquefaction residues of sewage sludge [J]. Bioresource Technology, 2011, 102(22): 10346- 10351. DOI: https://doi.org/10.1016/j.biortech.2011.08.117.
[26] YUAN Xing-zhong, HUANG Hua-jun, ZENG Guang-ming, LI Hui, Wang Jing-yu, ZHOU Chun-fei, ZHU Hui-na, PEI Xiao-kai, LIU Zhi-feng, LIU Zhan-tao. Total concentrations and chemical speciation of heavy metals in liquefaction residues of sewage sludge [J]. Bioresource Technology, 2011, 102(5): 4104-10. DOI: https://doi.org/10.1016/j.biortech. 2010.12. 055.
[27] ZHAI Yun-bo, CHEN Hong-mei, XU Bi-bo, XIANG Bo-bin, CHEN Zhong, Li Cai-ting, ZENG Guang-ming. Influence of sewage sludge-based activated carbon and temperature on the liquefaction of sewage sludge: Yield and composition of bio-oil, immobilization and risk assessment of heavy metals [J]. Bioresource Technology, 2014, 159: 72-79. DOI: https://doi. org/10.1016/j.biortech.2014.02.049.
[28] CHEN Hong-mei, ZHAI Yun-bo, XU Bi-bo, XIANG Bo-bin, ZHU Lu, QIU Lei, LIU Xiao-ting, LI Cai-ting, ZENG Guang-ming. Fate and risk assessment of heavy metals in residue from co-liquefaction of Camellia oleifera cake and sewage sludge in supercritical ethanol [J]. Bioresource Technology, 2014, 167: 578-581. DOI: https://doi.org/ 10.1016/j.biortech.2014.06.048.
[29] LENG Li-jian, LENG Song-qi, CHEN Jie, YUAN Xing-zhong, LI Jun, LI Kun, WANG Yun-pu, ZHOU Weng-guang. The migration and transformation behavior of heavy metals during co-liquefaction of municipal sewage sludge and lignocellulosic biomass [J]. Bioresource Technology, 2018, 259: 156-163. DOI: https://doi.org/ 10.1016/ j.biortech.2018.03.019.
[30] LENG Li-jian, YUAN Xing-zhong, HUANG Hua-jun, JIANG Hong-wei, CHEN Xiao-hong, ZENG Guang-ming. The migration and transformation behavior of heavy metals during the liquefaction process of sewage sludge [J]. Bioresource Technology, 2014, 167: 144-150. DOI: https:// doi.org/10.1016/j.biortech.2014.05.119.
[31] YUAN Xing-zhong, LENG Li-jian, HUANG Hua-jun, CHEN Xiao-hong, WANG Hou, XIAO Zhi-hua, ZHAI Yun-bo, CHEN Hong-mei, ZENG Guang-ming. Speciation and environmental risk assessment of heavy metal in bio-oil from liquefaction/pyrolysis of sewage sludge [J]. Chemosphere, 2015, 120: 645-652. DOI: https://doi.org/ 10.1016/ j.chemosphere.2014.10.010.
[32] SHAO Jian-guang, YUAN Xing-zhong, LENG Li-jian, HUANG Hua-jun, JIANG Long-bo, WANG Hou, CHEN Xiao-hong, ZENG Guang-ming. The comparison of the migration and transformation behavior of heavy metals during pyrolysis and liquefaction of municipal sewage sludge, paper mill sludge, and slaughterhouse sludge [J]. Bioresource Technology, 2015, 198: 16-22. DOI: https://doi.org/ 10.1016/j.biortech.2015.08.147.
[33] HUANG Hua-jun, YANG Ting, LAI Fa-yin, WU Guo-qiang. Co-pyrolysis of sewage sludge and sawdust/rice straw for the production of biochar [J]. Journal of Analytical and Applied Pyrolysis, 2017, 125: 61-68. DOI: https://doi.org/10.1016/ j.jaap. 2017.04.018.
[34] YANG Ting, HUANG Hua-jun, LAI Fa-yin. Pollution hazards of heavy metals in sewage sludge from four wastewater treatment plants in Nanchang, China [J]. Transactions of Nonferrous Metals Society of China, 2017, 27(10): 2249-2259. DOI: 10.1016/S1003-6326(17)60251-6.
[35] ZHAO Shou, FENG Cheng-hong, YANG Yi-ru, NIU Jun-feng, SHEN Zhen-yao. Risk assessment of sedimentary metals in the Yangtze Estuary: New evidence of the relationships between two typical index methods [J]. Journal of Hazardous Materials, 2012, 241-242: 164-172. DOI: https:// doi.org/10.1016/j.jhazmat.2012.09.023.
[36] IKEN A, EGIEBOR N O, NYAVOR K. Trace elements in water, fish and sediment from Tuskegee lake, southeastern USA [J]. Water, Air, and Soil Pollution, 2003, 149: 51-75. DOI: https://doi.org/10.1023/a:1025694315763.
[37] CHANAKA-UDAYANGA W D, VEKSHA A, GIANNNIS A, LISAK G, CHANG V W C, LIM T T. Fate and distribution of heavy metals during thermal processing of sewage sludge [J]. Fuel, 2018, 226: 721-744. DOI: https://doi.org/ 10.1016/j.fuel.2018.04.045.
[38] SHI Wan-sheng, LIU Chun-guang, SHU You-ju, FENG Chuan-ping, LEI Zhong-fang, ZHANG Zhen-ya. Synergistic effect of rice husk addition on hydrothermal treatment of sewage sludge: Fate and environmental risk of heavy metals [J]. Bioresource Technology, 2013, 149: 496-502. DOI: https://doi.org/10.1016/j.biortech.2013.09.114.
[39] GB/T 24600-2009. China’s Ministry of Housing and Urban-Rural Consruction. Disposal of sludge from municipal wastewater treatment plant―Quality of sludge used in land improvement [S]. (in Chinese)
[40] LIM M C H, AYOKO G A, MORAWSKA L, RISTOVSKI Z D, JAYARATNE E R. The effects of fuel characteristics and engine operating conditions on the elemental composition of emissions from heavy duty diesel buses [J]. Fuel, 2007, 86(12): 1831-1839. DOI: https://doi.org/10.1016/j.fuel.2006. 11.025
[41] YlLDlRlM G, TOKALlOGLU S. Heavy metal speciation in various grain sizes of industrially contaminated street dust using multivariate statistical analysis [J]. Ecotoxicology and Environmental Safety, 2016, 124: 369-376. DOI: https://doi.org/10.1016/j.ecoenv.2015.11.006.
[42] LIANG Xin, NING Xun-an, CHEN Guo-xin, LIN Mei-qing, LIU Jing-yong, WANG Yu-jie. Concentrations and speciation of heavy metals in sludge from nine textile dyeing plants [J]. Ecotoxicology and Environmental Safety, 2013, 98: 128-134. DOI: https://doi.org/10.1016/j.ecoenv.2013.09.012.
[43] FUENTES A, LLORENS M, SAEZ J, AGUILAR M I, ORTUNO J F, MESEGUER V F. Comparative study of six different sludges by sequential speciation of heavy metals [J]. Bioresource Technology, 2008, 99(3): 517-525. DOI: https://doi. org/10.1016/j.biortech.2007.01.025.
[44] SHI Wan-sheng, LIU Chun-guang, DING Da-hu, LEI Zhong-fang, YANG Ying-nan, FENG Chuang-ping, ZHANG Zhen-ya. Immobilization of heavy metals in sewage sludge by using subcritical water technology [J]. Bioresource Technology, 2013, 137: 18-24. DOI: https://doi.org/10.1016/ j.biortech.2013.03.106.
[45] NAZARI L, YUAN Zhong-shun, RAY M B, XU Chun-bao. Co-conversion of waste activated sludge and sawdust through hydrothermal liquefaction: Optimization of reaction parameters using response surface methodology [J]. Applied Energy, 2017, 203: 1-10. DOI: https://doi.org/10.1016/ j.apenergy.2017.06.009.
(Edited by HE Yun-bin)
中文导读
基于乙醇-水混合溶剂污泥液化过程中重金属的迁移和转化
摘要:污水厂污泥在乙醇-水混合溶剂中液化是制备生物油/生物炭的一种有前景的方法。乙醇和水联合使用对污泥液化过程中重金属的迁移和转化行为的影响是有关上述液化技术的安全性和清洁性方面的重要课题。本论文针对这一课题展开了研究,结果表明:纯乙醇作为液化溶剂时,重金属向生物炭迁移的比例更高;纯水作为液化溶剂时,生物炭中活性态重金属的比例则更低;与单一溶剂液化相比,乙醇-水共溶剂对重金属的分布和转化行为的影响呈现随机效应或平均效应。污泥在纯水中液化后,重金属的污染程度由生原污泥的重度污染(污染指数25.8)降低到生物炭的中度-重度污染(污染指数13.4),生态风险则由中等风险(风险指数164.5)降低到低风险(风险指数78.8)。纯乙醇作为液化溶剂时对重金属的污染程度和生态风险影响甚微。乙醇和水的联合使用具有和纯水类似的重金属钝化效应。污泥在乙醇-水混合溶剂中液化所得的生物炭中重金属的污染指数和风险指数分别是13.1~14.6(中度-重度污染)和79.3~101.0(低风险)。为了进一步控制基于乙醇-水混合溶剂污泥液化过程中重金属的污染,可以通过在污泥液化过程中引入常规的木质纤维素或藻类生物质(也即采用共液化技术)。
关键词:污泥;液化;乙醇-水混合溶剂;重金属;污染程度;生态风险
Foundation item: Project(21707056) supported by the National Natural Science Foundation of China; Project(20151BAB213024) supported by the Natural Science Foundation of Jiangxi Province, China; Project(GJJ14302) supported by the Scientific Research Fund of Jiangxi Provincial Education Department, China
Received date: 2018-08-11; Accepted date: 2019-01-29
Corresponding author: HUANG Hua-jun, PhD, Associate Professor; Tel: +86-791-83828028; E-mail: huanghuajun2004@126.com;HE Xiao-wu, PhD, Associate Professor; E-mail: he-xw@163.com; ORCID: 0000-0002-4104-9471

