Improvement of chromium biosorption through protoplast electrofusion between Candida tropicalis and Candida lipolytica
来源期刊:中南大学学报(英文版)2012年第6期
论文作者:何宝燕 尹华 杨峰 叶锦韶 彭辉 卢显妍 张娜
文章页码:1693 - 1701
Key words:chromium; biosorption; fusant; protoplast; electrofusion
Abstract: Protoplasts from Candida tropicalis and Candida lipolytica were fused under an optimized electrofusion (electrical pulse strength 6 kV/cm, pulse duration time 40 μs and pulse times 5) and then regenerated on YEPD media for achieving new genotypes with higher chromium loading capacity. A target fusant RHJ-004 was screened out by its chromium resistance and chromium-sorbing capacity tests for further research. The comparative study of applicability shows that the fusant has better performance than its parent strains in respect of solution pH, biomass concentration and chromium loading capacity. Especially for treating low concentration Cr(VI) (≤20 mg/L), above 80% chromium is sequestered from the aqueous phase at pH 1-9. Atomic force microscopy (AFM) visualizes the distribution of chromium on the binding sites of the cells, suggesting that the altered surface structure and intracellular constitutes of the fusant associate with its increased biosorption capacity. The rapid biosorption processes of chromium follow the Langmuir model well.
J. Cent. South Univ. (2012) 19: 1693-1701
DOI: 10.1007/s11771-012-1195-y![]()
HE Bao-yan(何宝燕)1, 2, YIN Hua(尹华)1, 2, YANG Feng(杨峰)1, 2, YE Jin-shao(叶锦韶)1, 2,
PENG Hui(彭辉)1, 2, LU Xian-yan(卢显妍)1, 2, ZHANG Na(张娜)1, 2
1. Department of Environmental Engineering, Jinan University, Guangzhou 510632, China;
2. Key Laboratory of Water/Soil Toxic Pollutants Control and Bioremediation of Guangdong Higher Education Institutes (Ji’nan University), Guangzhou 510632, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract: Protoplasts from Candida tropicalis and Candida lipolytica were fused under an optimized electrofusion (electrical pulse strength 6 kV/cm, pulse duration time 40 μs and pulse times 5) and then regenerated on YEPD media for achieving new genotypes with higher chromium loading capacity. A target fusant RHJ-004 was screened out by its chromium resistance and chromium-sorbing capacity tests for further research. The comparative study of applicability shows that the fusant has better performance than its parent strains in respect of solution pH, biomass concentration and chromium loading capacity. Especially for treating low concentration Cr(VI) (≤20 mg/L), above 80% chromium is sequestered from the aqueous phase at pH 1-9. Atomic force microscopy (AFM) visualizes the distribution of chromium on the binding sites of the cells, suggesting that the altered surface structure and intracellular constitutes of the fusant associate with its increased biosorption capacity. The rapid biosorption processes of chromium follow the Langmuir model well.
Key words: chromium; biosorption; fusant; protoplast; electrofusion
1 Introduction
In recent years, a variety of organisms (e.g. bacteria, alga, yeast and molds) have been tested for efficient adsorption or recovering of heavy metals from the polluted environment [1-2]. Compared with conventional chemical treatment technology, this kind of biosorption possesses many advantages such as high removal efficiency for low-concentration metal ions, strong selectivity towards specific metal ions, broad application range, and low secondary contamination [3-5]. However, the application of this promising technology is often restricted because of low metal-binding capacity and weak metal tolerance indigenous microorganisms. These strains can be developed by genetically modifying to increase high- affinity metal binding proteins or polypeptides. In addition to mutagenesis and gene recombination, protoplast fusion is also a possible alternative to generate new genotypes. Two or more heterologous cells in vitro, induced by electric field, can merge and form a hybrid cell through regeneration. Since the 1980s, electrofusion for breeding of various microorganisms has been widely used in many fields such as medicine and modern agriculture to produce new species because of its simplicity and high fusion efficiency [6].
Chromium is widely distributed as environmental pollutants coming from varying industries such as electroplating, chromate manufacturing, alloy reparation, metal cleaning and processing, leather tanning and wood preservation [7]. The oxidation states of chromium in these industrial effluents are primarily trivalent and hexavalent [8]. Hexavalent chromium poses health hazard on animals and humans, and chronic exposure to it may cause vomiting, severe diarrhea, hemorrhage and even cancer of digestive tract and lungs [9]. Therefore, safe transformation and/or removal of Cr(VI) from polluted environment have been a daunting problem [10].
Biological adsorbing materials sequester heavy metals from aqueous solutions by either metabolically mediated or physico-chemical uptake. The former refers to active transport across cell membrane, while the latter encompasses a number of metabolism-independent processes including physical and chemical adsorption, electrostatic interaction, ion exchange, chelation and micro precipitation that mainly take place on the cell surface [10].
In the present work, new genotypes were developed for the purpose of breeding strains of super chromium accumulator by protoplast electrofusion between Candida tropicalis (C. tropicalis) and Candida lipolytica (C. lipolytica) followed with efficient selection processes. To gain insight into possible alteration caused by electrofusion, a preferred fusant RHJ-004 was studied in respect of cell microstructure, chromium biosorption characteristics and the corresponding mechanisms in comparison with the parent strains. The biosorption behaviors were examined by popular adsorbing kinetic equations.
2 Materials and methods
2.1 Strains and media
In this work, Candida tropicalis (C. tropicalis), which has strong tolerance to high concentration chromium (2 000 mg/L), and Candida lipolytica (C. lipolytica) with high chromium binding capacity were used.
All media used were based on yeast culture media YEPD (pH 6.0) containing 20 g/L glucose, 10 g/L peptone and 10 g/L yeast extract. Sorbitol was added in YEPD to the final concentration of 0.8 mol/L as protoplasts regeneration medium. The peptone and yeast extract in YEPD were reduced to 2 g/L for the preparation of biosorbents.
2.2 Preparation of protoplasts
The fresh cells were inoculated into liquid YEPD medium and cultured for 8 h at 30 ℃ on a shaker. The biomass was harvested by centrifugation and washed three times with buffer PB (0.2 mol/L phosphate buffer solution, 0.8 mol/L sorbitol, pH 6.0). Afterwards, the cells experienced a 2 h enzymatic hydrolysis by 5 u/mL lyticase at 30 ℃, resulting in lysis of cell wall and release of protoplasts. Finally, the protoplasts were collected by centrifugation at 1 450g for 10 min and washed three times with buffer PB in order to get rid of residual enzyme.
2.3 Electric-induced fusion of protoplast
Equal volumes (100 μL) of protoplasts from C. lipolytica and C. tropicalis were mixed and then washed with PM solution (0.1 mmol/L CaCl2 and 0.8 mol/L mannitol) twice. The above prepared sample was injected into an electrode fusion chamber providing rectangular pulses. The electric-fused cells were allowed to recover in the chamber for 30 min at 37 ℃, then harvested, and washed once with PM solution prior to plating on Petri plates containing protoplasts regeneration medium. Pulse strength, pulse duration time and pulse times are crucial factors for efficient electroporation and regeneration of protoplast, and in turn, for attaining preferred fusants [11]. The survival ratio of protoplasts from C. lipolytica and C. tropicalis exposed to different pulses was examined to optimize electric-fusion conditions.
2.4 Screening fusion
The regenerated cells were spread onto Petri dishes containing YEPD medium. Circular filter papers (0.5 cm in diameter) saturated with 20 000 mg/L K2Cr2O7 solution were laid in the centre of the culture plates and thus chromium concentration gradients around the centre were formed by diffusion. After incubation at 30 ℃ for 2-3 d, different colonies appeared. Colonies that lived near the centre of filter papers indicating stronger tolerance to higher concentrations of chromium were selected, subcultured, and later tested for chromium removal. The testing conditions include: biomass 25 g/L, initial Cr(VI) concentration 20 mg/L, pH 2 and contact time 2 h. Seventeen fusants were picked out from the colonies. A preferred fusant RHJ-004 was selected for further biosorption characteristic study compared to parent strains.
2.5 Chromium biosorption capacity
The experiments were carried out in 250 mL triangular flasks with dry biosorbent mass of 0.36 g per 100 mL aqueous solution. The flasks, containing Cr(VI) solutions (5, 20, 50, 100 and 200 mg/L), were kept at 30 ℃ on a rotary shaker at 120 r/min with initial pH set at 1, 3, 5, 7, 9, 11 and 13. After shaking for 2 h, the mixtures were centrifuged and residual concentrations of Cr(VI) and Cr(III) in the supernatant were analyzed by 1,5-diphenylcarbazide spectrophotometric method. The reduction ratio of Cr(VI) and the removal ratio of total chromium were calculated using Eq. (1) and Eq. (2), respectively [12]. The influence of biosorbent dosage (0.72-4.32 g/L) and initial Cr(VI) concentration (5, 20, 50, 100 and 200 mg/L) on reduction and removal ratios were investigated. The equilibrium data for treating different concentrations of Cr(VI) (1, 3, 5, 10, 15, 20, 25 and 30 mg/L) were simulated by isotherm models (Langmuir, Freundlich and Dubinin-Radushkevich).
![]() (1)
(1)
where Qr stands for reduction ratio (%); ca and cb are initial and final Cr(VI) concentrations, respectively.
![]() (2)
(2)
where Qe represents removal ratio (%); co and ce are initial and final concentrations of total chromium, respectively.
The amount of heavy metal ions adsorbed at equilibrium, q (mmol/g), was calculated from the difference in metal concentration in the aqueous phase before and after biosorption, according to the following equation:
![]() (3)
(3)
where V is the volume of metal solution (L); co and ce are initial and final concentrations of total chromium (mmol/L), respectively; m is the mass of dry biosorbents (g).
2.6 Cells microstructure
After incubation in a solution with chromium concentration of 250 mg/L for 8 h, the cells were collected by centrifugation and rinsed three times with distilled water. Then, the cells were suspended in sterilized water and broken up by an ultrasonic pulverizer (power 900 W, working time 50 s, 200 times), followed by centrifugation (16 600g) to collect the cell inclusions. The cell surfaces and the cell inclusions were visualized by atomic force microscopy (AFM). The samples without chromium were served as control.
3 Results and discussion
3.1 Determination of electro-fusion condition
The strength and duration time are two main parameters for electric pulse. As can be seen in Fig. 1(a), under the pulses of 0-6 kV/cm, the survival rates of both C. lipolytica and C. tropicalis protoplasts exceed 70%, but as the pulse intensifies, nearly no protoplast can survive at 10 kV/cm. At constant pulse strength of 6 kV/cm, the survival rate of protoplasts decreases linearly with the prolonged duration time, as shown in Fig. 1(b). Over 50 μs exposure leads to the death of protoplasts by nearly 100%. At a lower pulse times below 5, as seen in Fig. 1(c), 75% of the protoplasts survives (strength 6 kV/cm and duration time 40 μs). In all, the survival rate of protoplasts shows negative relation with pulse strength, duration time and pulse times. Low survival rate indicates that protoplasts are broken down irreversibly, while high survival rate suggests ineffective exposure to pulse. In order to achieve high fusion efficiency, 70%-80% survival rate are selected for regeneration of fusants; thus, the optimal electro-fusion condition is set at voltage of 6 kV/cm, duration time of 40 μs, and pulse times of 5.
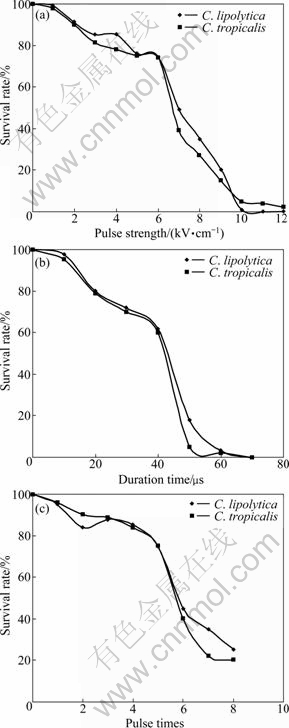
Fig. 1 Effect of pulse on survival rates of protoplasts: (a) Pulse strength; (b) Pulse duration time; (c) Pulse times
3.2. Comparison of chromium biosorption capacity between parents and fusant
3.2.1 Chromium removal capacity at different pH
Earlier studies on heavy metal biosorption have demonstrated that pH is the most important parameter [7]. Batch sorption experiments were performed at various pH (1.0, 3.0, 5.0, 7.0, 9.0, 11.0 and 13.0) by keeping all other experimental conditions constant (temperature, 30 ℃; agitation, 120 r/min; biosorbent, 0.36 g/100 mL; chromium concentration, 20 mg/L). Figure 2(a) shows that acidic condition (pH<7) favours Cr(VI) reduction and removal, and alkaline condition presents negative effect, which is also found in the study of WANG et al [13]. One explanation is that reduction reaction of Cr(VI) needs to consume a large quantity of H+ [14], as evidenced by the rise of solution pH after Cr(VI) reduction in acidic solution. At pH 1, Cr(VI) reduction and removal ratios by the parent strains C. lipolytica and C. tropicalis all exceed 80%, and nearly 100% removal is attained by the fusant RHJ-004. It is noticed that in neutral and alkaline solutions the fusant also exhibits better performance than the parent strains, and in the range of pH 1-9, its removal of chromium surpasses 80%, which means that the fusant has more favorable adaptability to the pH change. Another explanation is that, when Cr(VI) comes in contact with organic substances or reducing agents, especially in an acidic medium, Cr(VI) is easily or spontaneously reduced to Cr(III) because of its high redox potential value (above +1.3 V at standard condition) [15]. Meanwhile, Cr(III) has higher fat-soluble characteristic, and consequently, is more easily utilized by organism than Cr(VI). According to PARK et al, the mechanism of Cr(VI) bio-adsorption is not “anionic adsorption” but “adsorption coupled reduction” [16]. These results agree well with our previous studies [14] that Cr(VI) can be reduced to Cr(III) by extracellular secretions and then transported across cell membrane.
3.2.2 Chromium removal capacity by different concentrations of biomass
Biomass dosage is an important factor for the efficiency of heavy metal removal from wastewater. To find the most appropriate dosage, chromium reduction and removal efficiency were plotted against biosorbent concentration in Fig. 2(b). Apparently, with increasing biomass concentrations, reduction and removal ratios of Cr(VI) increase sharply when the concentrations are less than 1.44 g/L, then the increase of the ratios slows down, finally approaches a plateau at nearly 3.60 g/L. In comparison with the parent strains, the fusant RHJ-004 exhibits stronger chromium binding capacity. 80% Cr(VI) is removed from 1 L aqueous solution by 1.44 g RHJ-004, but the same removal performance requires 3.60 g parent strains.
3.2.3 Chromium removal capacity at different concentrations of Cr (VI)
Besides the solution pH, chromium concentration is another key factor shown to influence the equilibria and the kinetics of both biosorption and reduction. A series of biosorption experiments for dealing with different concentrations (5.0, 20.0, 50.0, 100.0 and 200.0 mg/L) of
Cr(VI) solutions at pH 2 were carried out at 30 ℃ and the results are shown in Fig. 2(c). For lower concentrations of Cr(VI) (<20 mg/L), all the strains (C. lipolytica, C. tropicalis and fusant RHJ-004) exhibit high reduction and removal ratios and a removal ratio of as high as 100% is achieved by the fusant when initial Cr(VI) concentration is 5 mg/L. As the initial chromium concentrations are elevated, the removal ratio of chromium decreases quickly; conversely, high chromium biosorption capacity is achieved because of ion diffusion from aqueous to solid phase, known as passive uptake. It is particularly worth mentioning that chromium loading capacity of RHJ-004 exceeds that of both the parent strains, not only at low concentrations of chromium but also at elevated levels. Even at high concentrations of Cr(VI) (>100 mg/L), RHJ-004 still demonstrates nearly 60% reduction and removal ratio, but as for the parent strains, only about 10% chromium is sequestered from the aqueous phase.
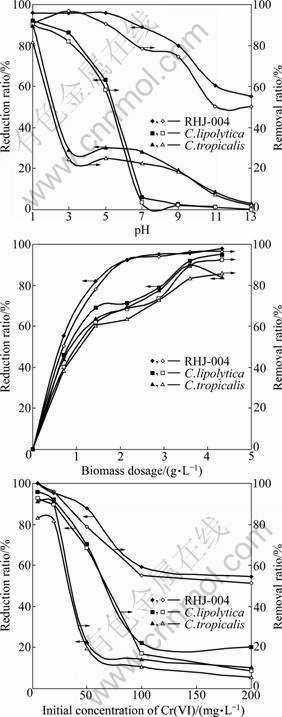
Fig. 2 Effect of biosorption conditions on Cr(VI) reduction and removal efficiency: (a) Initial pH of aqueous solution; (b) Biomass dosage; (c) Initial Cr(VI) concentration
Comparison between the Cr(VI) removal efficiency of the strains in this work and other adsorbents reported in the literatures are given in Table 1. The result indicates that the fusant RHJ-004 has better performance for treating low concentration Cr(VI) solution with high removal efficiency and broad pH application range.
3.3 Morphological details of biosorbent
3.3.1 Morphological details of cell surface
As a control, parent strain C. lipolytica was selected for exploring possible morphological changes of the fusant RHJ-004. As can be seen in Fig. 3, untreated cell surfaces of both the fusant and parent strain seem smooth. After loading chromium, many particles appear on the cell surface of RHJ-004, which possibly are extracellular secretions composed of polysaccharides, proteins, lipids and other biological macromolecules containing functional groups such as carboxyl, hydroxyl, phosphate, and amino group as heavy metal binding sites for sequestrating chromium ions from aqueous phase [21]. Our previous researches also confirmed that under the pressure of strong toxic chromium, some macromolecules such as protein and saccharides were produced as detoxification agents for the reduction of strong toxic Cr(VI) [14]. The “sandwich structure” of yeast possesses large specific area, capable of capturing and binding metal ions with active sites by electrostatic adherence, oxidation-reduction and coordination- chelation, etc. [13, 22].
Table 1 Comparison of removal efficiency of low concentration Cr(VI) by different biomaterials
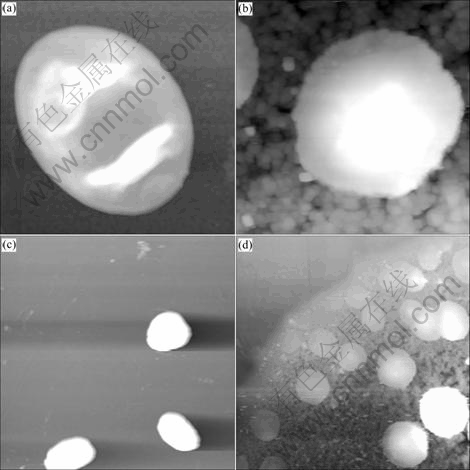
Fig. 3 AFM images of cell surface of C. tropicalis (scanning range 7 μm) and RHJ-004 (scanning range 30 μm): (a) C. tropicalis; (b) C. tropicalis after biosorption; (c) Fusant RHJ-004; (d) Fusant RHJ-004 after biosorption
3.3.2 Morphological details of cell inclusions
Cell inclusions of C. tropicalis and RHJ-004 separated from cells by ultrasonication were visualized under AFM for the analysis of their morphological differences and the related organic-metal bonding states. As can be seen in Fig. 4(a) and Fig. 4(b), the inclusions of native C. tropicalis disperse uniformly, while after saturation with chromium, they agglomerate to particles of about 0.5 μm in diameter. For RHJ-004, the inclusions loading high concentration chromium appear netlike and peak in the middle, different from layer-like image before biosorption (Figs. 4(c) and 4(d)). The aggregation of these inclusions shows that the invasion of chromium inside the cells alters the macromolecule configuration and the resulting reticular structure is more favorable for capturing chromium than the orbicular, involving in the higher intracellular accumulation potential of RHJ-004. By binding with large amounts of chromium ions, those macromolecular substances concentrate and form the reticular bulging in the middle. It is probably due to the acidic conditions that intracellular amino groups ionize and form positively charged surface. Negative chromic ions are adsorbed on these groups, where biological macromolecules cross linkage, and thus form large netlike structure of Cr-macromolecule.
3.4 Adsorption isotherms
Although the above experimental studies offer some evidences of biosorption characteristics and mechanisms, analysis of equilibrium data is important for developing an equation that can be used to compare different biosorbents under different operational conditions and to design and optimize an operating procedure [23]. Among the isotherm models, Langmuir, Freundlich, and Dubinin- Radushkevich models are widely used to examine the correlation between biosorption capacity and adsorbent concentration at equilibrium [24-25]. To describe the distribution of the solute in solid and liquid phases under equilibrium conditions, it is necessary to express the amount of solute absorbed by unit mass of sorbent, qe (mmol/g), as a function of the residual equilibrium concentration, ce (mmol/L), of solute remaining in solution. In this work, to obtain equilibrium data, initial chromium concentrations vary in the range of 1-30 mg/L while the biomass (3.6 g/L), reaction temperature (30 ℃), and reaction time (30 min) in each reaction system are kept constant. The resulted plots in Fig. 5 show that the chromium biosorption capacity by the strains (RHJ-004, C. lipolytica and C. tropicalis) goes up as initial chromium concentration increases until the saturation of the binding sites, which is due to the driving forces induced by concentration gradients of Cr(VI) from 0.019 to 0.577 mmol/L. It is noticed that at different equilibrium concentrations of chromium, the biosorption capacity of RHJ-004 is significantly higher than that of the parent strains.
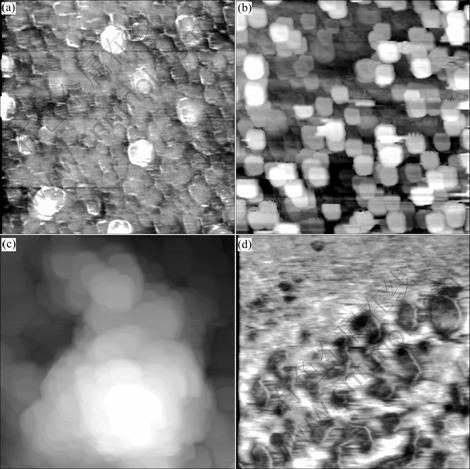
Fig. 4 AFM images of cell inclusions of C. tropicalis (scanning range 4 μm) and fusant RHJ-004 (scanning range 5 μm): (a) C. tropicalis; (b) C. tropicalis after biosorption; (c) Fusant RHJ-004; (d) Fusant RHJ-004 after biosorption
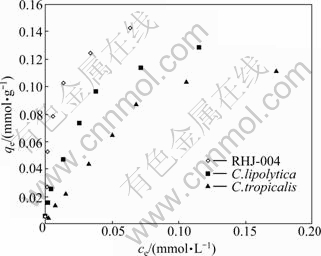
Fig. 5 Equilibria of chromium biosorption by different biosorbents
3.4.1 Langmuir isotherm
The Langmuir isotherm, a single-component adsorption model, relates the coverage of molecules on a solid surface at a fixed temperature. All surface sites are alike and a molecule to be adsorbed on a given site is independent of the occupancy of its neighboring sites. Based on this, a kinetic principle (rate of adsorption and desorption from the surface is equal), Langmuir Eq. (3) can be written in the following form [26]:
![]() (3)
(3)
where KL (L/g) is the Langmuir constant related to the free energy of biosorption.
3.4.2 Freundlich isotherm
The Freundlich expression is an equation based on heterogeneous surfaces suggesting that binding sites are not equivalent and/or independent. The Freundlich model is described by the following equation:
![]() (4)
(4)
where KF is the Freundlich constant related to biosorption capacity of biosorbent and n is Freundlich exponent indicating the biosorption intensity [17].
3.4.3 Dubinin-Radushkevich (D-R) isotherm
Since the Freundlich and Langmuir isotherm models do not provide any insight into the characteristics of biosorbents, D-R isotherm model is applied to testing the equilibrium data and the mathematical expression is as follows [27]:
![]() (5)
(5)
where ε=RTln(1+1/ce) (Polanyi potential), β is the constant related to the biosorption energy, R is the gas constant (8.314 kJ/mol) and T is the absolute temperature (K).
Polanyi sorption theory assumes that the fixed volume of sorption space is close to the sorbent surface and existence of sorption potential over these spaces. The mean free energy of biosorption (E) can be calculated from Eq. (6):
![]() (6)
(6)
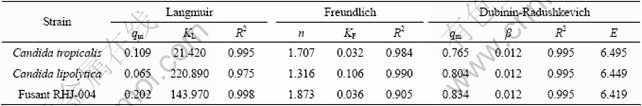
The fitted isotherm model constants and R2 values are included in Table 2. All equations simulate the data reasonably well with R2 values ranging from 0.905 to 0.998. But the best fit is obtained with the Langmuir isotherm model by fusant RHJ-004 and the high correlation coefficient (R2=0.998) implies that the adsorption of Cr(VI) is close to single-component adsorption. The maximum amount of metal absorbed on RHJ-004 (0.202 mmol/g) is far higher than that of C. lipolytica (0.065 mmol/g) and C. tropicalis (0.109 mmol/g). Freundlich constants KF of RHJ-004, C. lipolytica and C. tropicalis are 0.036, 0.106 and 0.032, respectively; while values of n are 1.873, 1.316 and 1.707 (Table 2). For RHJ-004, the magnitude of n indicates its stronger binding capacity with Cr. Among these models, Dubinin-Radushkevich model represents all the experimental data with relation coefficient (R2) of 0.995 and the corresponding biosorption capacity reaches 0.834 mmol/g (RHJ-004), 0.804 mmol/g (C. lipolytica) and 0.765 mmol/g (C. tropicalis). The magnitude of E is useful for estimating the mechanism of biosorption process and it is found to be 6.419, 6.449 and 6.495 kJ/mol for RHJ-004, C. lipolytica and C. tropicalis, respectively. The low biosorption free energy (< 8 kJ/mol) suggests that the process proceeds physically [11] and it accords well with the Langmuir model simulation result. Overall, the fusant has achieved stronger chromium binding and adsorbing capacity than the parent strains.
Table 2 Comparison of Langmuir, Freundlich and Dubinin-Radushkevich constants
4 Conclusions
1) C. lipolytica and C. tropicalis are utilized for generating novel genotypes to improve chromium biosorption capacity by protoplasts electrofusion. The resulted fusant achieves obvious advantages over the parent strains in respect of elevated chromium biosorption capacity, less pH-dependent and lower biosorbent dosage, and exhibits more potential than other reported adsorbents in literature for advanced treatment of chromium containing raw water. A good example is presented for generating new functional microbes to treat hazardous waste and recover contaminated soil and water body.
2) In the rapid biosorption processes (about 30 min), the Langmuir model simulates the biosorption equilibrium well and the cell surface uptake plays vital role in sequestering chromium from aqueous solutions.
3) The increased chromium loading capacity of the fusant may be attributed to its altered microstructure by electrofusion, as evidenced by the AFM images.
Acknowledgements
The authors would like to sincerely thank Professor Paul CHIEN from University of San Francisco for his helpful advice on the manuscript.
References
[1] AKAR A, CELIK S, AKAR S T. Biosorption performance of surface modified biomass obtained from Pyracantha coccinea for the decolorization of dye contaminated solutions [J]. Chemical Engineering Journal, 2010, 160(2): 466-472.
[2] KUMAR R, SINGH R, KUMAR N, BISHNOI K, BISHNOI N R. Response surface methodology approach for optimization of biosorption process for removal of Cr(VI), Ni(II) and Zn(II) ions by immobilized bacterial biomass sp. Bacillus brevis [J]. Chemical of Engineering Journal, 2009, 146(3): 401-407.
[3] SHENG Li, XIA Jin-lian, HE Huan, NIE Zhen-yuan, QIU Guan-zhou. Biosorption mechanism of Cr(VI) onto cells of synechococcus sp. [J]. Journal of Central South University of Technology, 2007, 14(2): 157-162.
[4] ZENG Xiao-xi, TANG Jian-xin, LIU Xue-duan, JIANG Pei. Isolation, identification and characterization of cadmium-resistant pseudomonas aeruginosa stains E1 [J]. Journal of Central South University of Technology, 2009, 16(3): 416-421.
[5] SARI A, ULUOZL? ? D, T?ZEM M. Equilibrium, thermodynamic and kinetic investigations on biosorption of arsenic from aqueous solution by algae (Maugeotia genuflexa) biomass [J]. Chemical Engineering Journal, 2011, 167(1): 155-161.
[6] TERPITZ U, RAIMUNDA D, WESTHOFF M, SUKHORUKOV V L, BEAUG? L, BAMBERG E, ZIMMERMANN D. Electrofused giant protoplasts of Saccharomyces cerevisiae as a novel system for electrophysiological studies on membrane proteins [J]. Biochimica et Biophysica. Acta (BBA)-Biomembranes, 2008, 1778(6): 1493-1500.
[7] AKAR T, KAYNAK Z, ULUSOY S, YUVACI D, OZSARI G, AKAR S T. Enhanced biosorption of nickel(II) ions by silica-gel- immoblized waste biomass: Biosorption characteristics in batch and dynamic flow mode [J]. Journal of Hazardous Materials, 2009, 163(2/3): 1134-1141.
[8] ARANDA-GARC?A E, NETZAHUATL-MU?OZ A R, CRISTIANI-URBINA M D C, MORALES-BARRERA L, PINEDA- CAMACHO G, CRISTIANI-URBINA E. Bioreduction of Cr(VI) and chromium biosorption by acorn shell of Quercus crassipes humb. & bonpl [J]. Journal of Biotechnology, 2010, 150(Supplement 1): 228.
[9] SUN Xue-fei, LIU Chuang-yong, MA Yue, WANG Shu-gang, GAO Bao-yu, LI Xiao-ming. Enhanced Cu(II) and Cr(VI) biosorption capacity on poly(ethylenimine) grafted aerobic granular sludge [J]. Colloids and Surfaces B: Biointerfaces, 2011, 82(2): 456-462.
[10] SINGHA B, DAS S K. Biosorption of Cr(VI) ions from aqueous solutions: Kinetics, equilibrium, thermodynamics and desorption studies [J]. Colloids and Surfaces B: Biointerfaces, 2011, 84(1): 221-232.
[11] TRONTELJ K, REBERSEK M, KANDUSER M, SERBEC V C. Optimization of bulk cell electrofusion in vitro for production of human-mouse heterophybridoma cells [J]. Bioelectrochemistry, 2008, 74(1): 124-129.
[12] ANAYURT R A, SARI A, TUZEN M. Equilibrium, thermodynamic and kinetic studies on biosorption of Pb(II) and Cd(II) from aqueous solution by macrofungus (Lactarius scrobiculatus) biomass [J]. Chemical Engineering Journal, 2009, 151(1/2/3): 255-261.
[13] WANG Xue-song, TANG Ye-ping, TAO Sheng-rong. Kinetics, equilibrium and thermodynamic study on removal of Cr(VI) from aqueous solutions using low-cost adsorbent alligator weed [J]. Chemical Engineering Journal, 2009, 148(2/3): 217-225.
[14] YIN Hua, HE Bao-yan, LU Xian-yan, PENG Hui, YE Jin-shao, YANG Feng. Imporvement of chromium biosorption by UV-HNO2 cooperative mutagenesis in Candida utilis [J]. Water Research, 2008, 42(14): 3981-3989.
[15] BL?ZQUEZ G, HERN?INZ F, CALERO M, MART?N-LARA M A, TENORIO G. The effect of pH on the biosorption of Cr(III) and Cr(VI) with olive stone [J]. Chemical Engineering Journal, 2009, 148(2/3): 473-470.
[16] PARK D, YUN Y S, PARK J M. Mechanisms of the removal of hexavalent chromium by biomaterials or biomaterial-based activated carbons [J]. Journal of Hazardous Materials, 2006, 137(2): 1254-1257.
[17] LI Jian-ping, LIN Qing-yu, ZHANG Xue-hong, YAN Y. Kinetic parameters and mechanisms of the batch biosorption of Cr(VI) and Cr(III) onto Leersia hexandra Swartz biomass [J]. Journal of Colloid and Interface Science, 2009, 333(1): 71-77.
[18] GAO Hui, LIU Yun-guo, ZENG Guang-ming, XU Wei-hua, LI Ting, XIA Wen-bin. Characterization of Cr(VI) removal from aqueous solutions by a surplus agricultural waste-Rice straw [J]. Journal of Hazardous Materials, 2008, 150(2): 446-452.
[19] J?COME-PILCO C R, CRISTIANI-URBINA E, FLORES- COTERA L B, VELASCO-GARC?A R, PONCE-NOYOLA T, CA?IZARES-VILLANUEVA R O. Continuous Cr(VI) removal by Scenedesmus incrassatulus in an airlift photobioreactor [J]. Bioresource Technology, 2009, 100(8): 2388-2391.
[20] ERTUGAY N, BAYHAN Y K. Biosorption of Cr(VI) from aqueous solutions by biomass of Agaricus bisporus [J]. Journal of Hazardous Materials, 2008, 154(1/2/3): 432-439.
[21] COMTE S, GUIBAUD G, BAUDU M. Biosorption properties of extracellular polymeric substances (EPS) towards Cd, Cu and Pb for different pH values [J]. Journal of Hazardous Materials, 2008, 151(1): 185-193.
[22] AMINI A, YOUNESI H, BAHRAMIFAR N. Statistical modeling and optimization of the cadmium biosorption process in an aqueous solution using Aspergillus niger [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2009, 337(1/2/3): 67-73.
[23] GUPTA S, KUMAR D, GAUR J P. Kinetic and isotherm modeling of lead(II) sorption onto some waste plant materials [J]. Chemical Engineering Journal, 2009, 148(2/3): 226-233.
[24] NUHOGLU Y, MALKOC E. Thermodynamic and kinetic studies for environmentaly friendly Ni(II) biosorption using waste pomace of olive oil factory [J]. Bioresource Technology, 2009, 100(8): 2357-2380.
[25] WON S W, YUN H J, YUN Y S. Effect of pH on the binding mechanisms in biosorption of Reactive Orange 16 by Corynebacterium glutamicum [J]. Journal of Colloid and Interface Science, 2009, 331(1): 83-89.
[26] BHATTI H N, KHALID R, HANIF M A. Dynamic biosorption of Zn(II) and Cu(II) using pretreated Rosa gruss an teplitz (red rose) distillation sludge [J]. Chemical Engineering Journal, 2009, 148(2/3): 434-443.
[27] FEBRIANTO J, KOSASIH A N, SUNARSO J, JU Y H. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies [J]. Journal of Hazardous Materials, 2009, 162(2/3): 616-645.
(Edited by HE Yun-bin)
Foundation item: Project(NSFC-GDNSF U0933002) supported by the Joint Funds of the National Natural Science Foundation of China and the Natural Science Foundation of Guangdong Province, China; Project(50978122) supported by the National Natural Science Foundation of China
Received date: 2011-04-07; Accepted date: 2011-05-31
Corresponding author: YIN Hua, Professor, PhD; Tel: +86-20-85220564; Fax: +86-20-85226615; E-mail: thyin@jnu.edu.cn

