文章编号:1004-0609(2013)06-1775-08
中药渣和麦麸对模拟矿山酸性废水中Cu2+的吸附
陈月芳,曹丽霞,林 海,董颖博,程 皝,霍汉鑫
(北京科技大学 土木与环境工程学院,北京 100083)
摘 要:研究溶液pH值、Cu2+初始浓度、吸附时间、吸附剂投加量及温度对中药渣和麦麸吸附模拟矿山酸性废水中较高浓度Cu2+的影响。结果表明,随pH值的升高中药渣和麦麸对Cu2+的吸附量均增大;吸附剂最佳投加量为10 g/L;吸附过程更好地符合拟二级动力学模型;在pH值为3时,二者的最大吸附量分别为14.03和7.34 mg/g,吸附平衡符合Langmuir等温线方程;热力学研究表明,二者对Cu2+的吸附为非自发的放热反应。Zeta电位显示中药渣和麦麸在水溶液中均带负电,能够以静电引力吸附Cu2+;红外光谱分析表明,中药渣吸附Cu2+的官能团主要为羟基、羧基、酰胺基和酯基等,而麦麸吸附Cu2+的主要官能团为羟基、酰胺基和硅氧基等。
关键词:矿山酸性废水;中药渣;麦麸;生物吸附
中图分类号:X703.1 文献标志码:A
Adsorption of Cu2+ from simulated acid mine drainage by herb-medicine residues and wheat bran
CHEN Yue-fang, CAO Li-xia, LIN Hai, DONG Ying-bo, CHENG Huang, HUO Han-xin
(1. School of Civil and Environmental Engineering, University of Science and Technology Beijing, Beijing 100083, China)
Abstract: Effective parameters including solution pH, initial Cu2+ concentration, contact time, mass of adsorbents and temperature were studied to assess the biosorption of high Cu2+ ions from simulated acid mine drainage by herb-medicine residues and wheat bran. The results show that biosorption capacities of both of them increase with the raising solution pH; the addition of adsorbents is optimized to be 10 g/L; pseudo-second-order model is more suitable for biosorption processes. The maximum biosorption capacities are 14.03 and 7.34 mg/g, respectively at solution pH value of 3; Langmuir model is observed to fit well. Thermodynamics analysis indicates that biosorption processes of herb-medicine residues and wheat bran are exothermic and unspontaneous. By means of Zeta potentials, it is found that both herb-medicine residues and wheat bran bear negative charges in solution, which can adsorb Cu2+ by electrostatic pull. For FT-IR spectrum, the results indicate that the functional groups of herb-medicine residues are hydroxyl, carboxyl, amide and ester, while the functional groups of wheat bran are hydroxyl, amide and silicon-oxygen groups.
Key words: acid mine drainage; herb-medicine residues; wheat bran; biosorption
矿山酸性废水pH值较低,常在2~4左右,且含有Cu2+、Pb2+、Zn2+、Cd2+、As3+和Ni2+等多种重金属离子,其中Cu2+浓度甚至高达503 mg/L[1]。由于重金属离子能在食物链中转移并富集,进而危害人畜健康,因此,从矿山酸性废水中去除重金属离子是一个非常重要的研究课题[2]。
目前,矿山酸性废水的处理方法主要有化学沉淀、离子交换、膜分离以及吸附法等。这些方法在一定程度上能有效去除重金属离子,但也存在着成本高、化学药剂消耗量大、能耗高并产生有害泥渣等缺点。近年来,利用工农业废弃生物质果壳[3-4]、锯末[5-6]、树皮[7-8]、稻壳[9-10]和甘蔗渣[11-12]等来吸附重金属离子引起了人们的极大关注。由于该法具有吸附量大、效率高、操作简单、吸附剂来源广泛、成本低以及能够以废治废等众多优点,因而成为具有发展前景的矿山酸性废水处理方法。
我国作为中药生产大国和主要的小麦生产国,每年产生大量的中药渣和麦麸,其常见资源化途径是用作肥料、饲料和生产食用菌的原材料,经济附加值较低。由于中药渣和麦麸富含纤维素、多糖和蛋白质等有机物质,而这些有机物含有羟基、羧基等官能团,能够结合重金属离子[7],因此,可考虑作为生物吸附剂来处理重金属废水。韦平英等[13]研究了板蓝根药渣对人工配制的低浓度含铅废水的吸附特性,发现板蓝根药渣能快速、大量地吸附铅,对低浓度的铅溶液吸附率更高,吸附速度更快。WANG等[14]用麦麸来吸附Cu2+ (其浓度低于120 mg/L),结果发现,在pH 5、吸附剂投加量4 g/L的情况下,麦麸对Cu2+的最大吸附量为6.85 mg/g。然而,目前人们对利用中药渣和麦麸,特别是中药渣来处理低pH值、高重金属离子浓度的矿山酸性废水的试验研究还较少见,因此,本文作者对利用中药渣和麦麸吸附模拟矿山酸性废水中较高浓度Cu2+进行了试验研究,这对开发新型环保生物吸附剂、提高中药渣和麦麸的资源化利用率具有重大的意义,同时为其在矿山酸性含铜废水处理中的运用提供一定的理论依据。
1 实验
1.1 试验材料与药剂
中药渣来自某药厂炼制蜂王浆后的残渣,由大枣、枸杞、党参及五味子组成。麦麸购买于北京某公司。样品采集后,立即风干。在80 ℃下烘至恒量,其含水率分别为7.36%和8.01%(质量分数)。然后碾碎,过孔径450 μm筛,装入聚乙烯袋中,放入干燥器中备用。
试剂CuSO4·5H2O、H2SO4和NaOH均为分析纯。
1.2 试验方法及仪器
取200 mL一系列浓度的Cu2+溶液分别置于500 mL锥形瓶中,用H2SO4或NaOH调节溶液的pH值,分别投加一定量的中药渣和麦麸,然后在一定温度、140 r/min条件下恒温振荡一定时间,并设置空白对照组。吸附反应结束后用G3砂芯漏斗进行抽滤,取滤液,测定滤液中剩余Cu2+浓度。
试验仪器如下:HZQ-F160恒温振荡培养箱,UNICO UV-2100紫外可见分光光度计,Zeta plus电位分析仪,Nicolet Nexus 670型红外光谱分析仪。
1.3 测试及评价方法
溶液中Cu2+浓度采用改进的BCO法[15]进行测定,试验所得标准曲线如图1所示。

图1 Cu2+吸附标准曲线
Fig. 1 Standard curve of Cu2+ adsorption
Cu2+的去除效果由材料吸附量qt(mg/g)和离子去除率r(%)这两个指标来度量,其计算式如下:
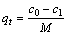 (1)
(1)
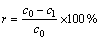 (2)
(2)
式中:c0为初始重金属离子浓度,mg/L;c1为剩余重金属离子浓度,mg/L;M为吸附材料投加量,g/L;
2 结果与讨论
2.1 pH值的影响
pH值是影响生物吸附的重要因素。在30 ℃、140 r/min、Cu2+初始浓度为150 mg/L、吸附剂投加量为5 g/L、吸附时间为4 h的条件下,考察溶液pH值在1.5~5.3(Cu2+开始沉淀的pH值为5.51)之间变化时对吸附Cu2+的影响,结果如图2所示。

图2 溶液pH值对Cu2+吸附的影响
Fig. 2 Effect of solution pH on biosorption of Cu2+
从图2可看出,中药渣和麦麸对Cu2+的吸附量均随着pH值的升高而增大。在pH值为1.5左右时,两种材料对Cu2+几乎无吸附作用;当pH值在2~4之间时,吸附量显著增大,中药渣的吸附量由2.32 mg/g增加到11.16 mg/g,麦麸的吸附量由1.12 mg/g增加到11.12 mg/g;当pH值超过4时,吸附量趋于平缓。
此外,在pH<4时,中药渣对Cu2+的吸附效果要明显优于麦麸的,且二者吸附量的差距随着pH值的升高先增大后缩小;当pH值超过4时,二者吸附量的差距不明显。这可能是由于吸附剂表面官能团的种类与数量的不同[14],如果吸附剂表面含有较多的能在较低pH值下就能与重金属离子有效结合的官能团,则该吸附剂在较低pH值下就能相对较好地吸附重金属离子。
由于实际矿山酸性废水pH值常在2~4之间,因此,结合试验结果将后续试验pH值均设为3。
2.2 Cu2+初始浓度的影响
矿山酸性废水中重金属离子的浓度常在每升几毫克到几百毫克的范围内变化,因此,研究了Cu2+初始浓度为20~420 mg/L、pH值为3、吸附剂投加量为5 g/L、吸附时间为4 h、吸附温度为30 ℃时,中药渣和麦麸对Cu2+的吸附,结果如图3所示。
由图3可看出,随着Cu2+初始浓度的增大,中药渣和麦麸的吸附量均相应增大。低浓度时,二者对 Cu2+的吸附效果相差不大;当Cu2+浓度大于100 mg/L时,中药渣对Cu2+的吸附效果明显优于麦麸的。分析其原因可能是,低浓度时二者可供利用的吸附位点均相对较多,所以其吸附效果差不多;在高浓度时,随着吸附过程的进行,二者的吸附位点陆续被占据,而在pH值影响试验中已得出中药渣在pH值为3的情况下其吸附量高于麦麸的,因此,中药渣的吸附量相对较大。
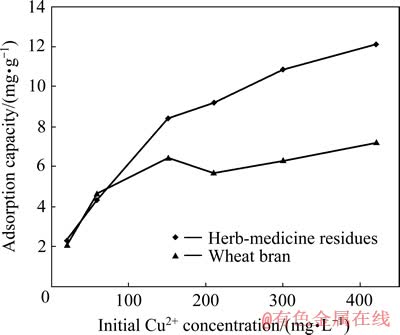
图3 Cu2+初始浓度对Cu2+吸附的影响
Fig. 3 Effect of initial Cu2+ concentration on biosorption of Cu2+
2.3 吸附时间的影响
研究吸附时间对实际废水处理反应器的设计及过程优化具有重大意义,较快的吸附平衡时间有利于生产实践。在pH值为3、吸附剂投加量为5 g/L、Cu2+浓度为150 mg/L、吸附温度为30 ℃时,不同吸附时间下的吸附效果如图4所示。
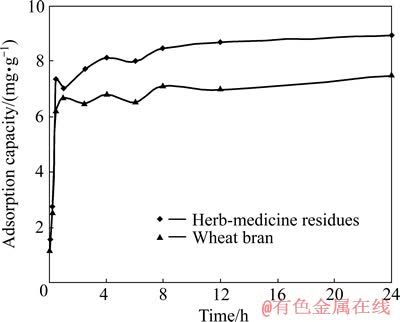
图4 吸附时间对Cu2+吸附的影响
Fig. 4 Effect of contact time on biosorption of Cu2+
由图4可看出,中药渣和麦麸在吸附的前0.5 h内吸附速率很快,对Cu2+的吸附量分别达到7.36和6.23 mg/g,基本达到吸附平衡;当吸附时间超过0.5 h以后,吸附速率缓慢,吸附量随时间的变化不再明显。这种快速吸附平衡为其工业化连续处理废水提供了可能。为保证充分吸附,后续试验吸附时间选取2 h。
2.4 温度的影响
温度是影响生物吸附的又一重要因素。在pH值为3、吸附剂投加量为5 g/L、Cu2+浓度为150 mg/L、吸附时间为2 h的条件下,考察中药渣和麦麸在温度20~40 ℃之间时对Cu2+的吸附,结果如图5所示。
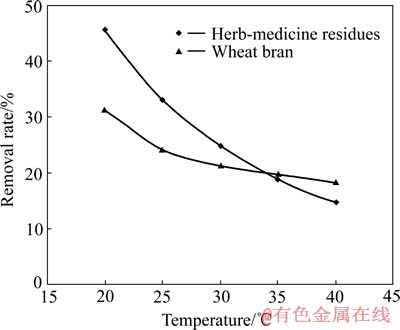
图5 温度对Cu2+吸附的影响
Fig. 5 Effect of temperature on biosorption of Cu2+
由图5可知,在20 ℃时中药渣和麦麸对Cu2+的去除率分别为45.73%和31.47%,之后去除率随着温度的升高而不断降低,中药渣降低了31%,麦麸降低了13.11%。这说明低温有助于二者对Cu2+的吸附,而高温不利于吸附,这可能是因为静电作用放热[16],从而导致因静电作用吸附的Cu2+量减少。此外,从二者去除率的降幅上也可推断出,静电吸附对中药渣吸附Cu2+的贡献要大于对麦麸吸附Cu2+的贡献。
2.5 中药渣和麦麸投加量影响
为了确定中药渣和麦麸的最佳投加量,研究了二者的投加量从2.5 g/L增大至15 g/L时,Cu2+去除率的变化,结果如图6所示。其他试验条件如下:pH值3,Cu2+浓度150 mg/L,吸附时间2 h,吸附温度30 ℃。
从图6可看出,中药渣和麦麸对Cu2+的去除率均随着投加量的增加而增大,在投加量为10 g/L时,去除率分别达到51.5 %和38.4 %;继续增大投加量至15 g/L,中药渣对Cu2+的去除率仅增加了3.3%,而麦麸对Cu2+的去除率却下降了4.75%。一般来说,随着吸附剂投加量的增大,重金属离子的去除率也会增大,但若吸附剂投加过量还可能使去除率下降,因为投加过量会导致吸附剂粒子团聚,减小吸附剂表面积[17]。因此,中药渣和麦麸的最佳投加量均取为10 g/L。
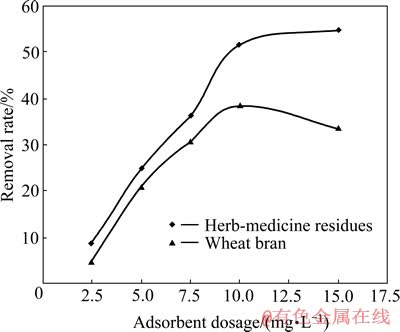
图6 吸附剂投加量对Cu2+吸附的影响
Fig. 6 Effect of adsorbent dosage on biosorption of Cu2+
2.6 吸附平衡
运用Langmuir及Freundlich等温线方程来分析中药渣和麦麸对Cu2+的吸附,所得等温吸附常数如表1所列。
表1 中药渣和麦麸对Cu2+的等温吸附常数
Table 1 Isotherm parameters for biosorption of Cu2+ on herb-medicine residues and wheat bran
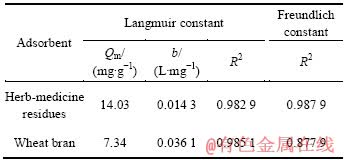
从表1可看出,中药渣对Langmuir方程的拟合系数R2为0.982 9,略小于采用Freundlich方程拟合的R2值,这说明中药渣对Cu2+的吸附同时存在单层及多层吸附,吸附机制较复杂;而麦麸对Langmuir方程的拟合系数R2为0.985 1,明显优于Freundlich方程,表明麦麸对Cu2+的吸附是以单分子层吸附为主。总体来说,二者对 Cu2+的吸附均符合Langmuir等温线方程。
此外,也可看出,中药渣的吸附常数b小于麦麸的吸附常数,表明中药渣与Cu2+的结合稳定性差,更容易被脱附下来[18],这与温度试验得到的现象相一致。
通过Langmuir方程拟合出中药渣和麦麸对Cu2+的最大吸附量Qm分别为14.03和7.34 mg/g,将其与其他一些常见生物材料对Cu2+的最大吸附量相比较,结果见表2。
表2 不同吸附材料对Cu2+的最大吸附量比较
Table 2 Comparison of maximum biosorption capacity by Cu2+ of various biosorbents
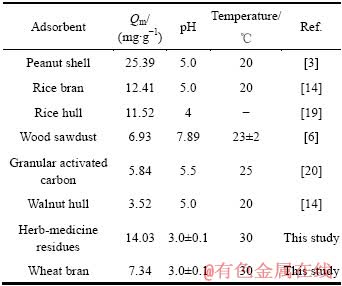
由表2可看出,其他生物质材料研究的均是在pH值大于4的情况下对Cu2+的最大吸附量,而中药渣和麦麸对Cu2+的最大吸附量却是在pH值为3左右时得出的,因此,通过对比可知,药渣和麦麸作为新型吸附剂在处理低pH值废水方面具有一定的竞争潜力。
2.7 吸附动力学
分别用拟一级动力学模型、拟二级动力学模型和粒子内部扩散模型[5, 7],如式(3)~(5)所示,对中药渣和麦麸吸附Cu2+进行动力学拟合,结果见表3。
 (3)
(3)
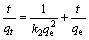 (4)
(4)
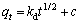 (5)
(5)
式中:t为吸附时间,min;qt为t时刻的吸附量,mg/g;qe为平衡吸附量,mg/g;k1为拟一级吸附速率常数,min-1;k2为拟二级吸附速率常数,g/(mg·min);kd为内部扩散速率常数,mg/(L·min0.5)。
表3 中药渣和麦麸吸附Cu2+的动力学参数
Table 3 Kinetics parameters for biosorption of Cu2+ by herb-medicine residues and wheat bran

从表3可看出,二者拟一级动力学模型的相关系数远小于其拟二级动力学模型的相关系数,其中,拟二级动力学模型相关系数均达到0.99以上,相关性较好,从而说明可用拟二级动力学模型描述中药渣和麦麸吸附Cu2+的动力学过程,且吸附速率被化学吸附所控制[17, 21]。此外,粒子内部扩散模型常数c并不等于0,表明粒子内部扩散并不是唯一的速控步骤,还应考虑膜扩散。
2.8 吸附热力学
吉布斯自由能变(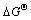 )、焓变(
)、焓变( )和熵变(
)和熵变(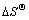 )被常用来分析吸附过程的自发性,按式(6)~(8)进行计算:
)被常用来分析吸附过程的自发性,按式(6)~(8)进行计算:
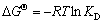 (6)
(6)
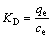 (7)
(7)
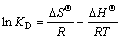 (8)
(8)
式中:R为摩尔气体常数,8.314 J/(mol·K);T为热力学温度,K;ce为平衡时剩余重金属离子浓度,mg/L;KD为扩散系数。
中药渣和麦麸吸附Cu2+的热力学参数如表4所列。
表4 中药渣和麦麸吸附Cu2+的热力学参数
Table 4 Thermodynamics parameters for biosorption of Cu2+ by herb-medicine residues and wheat bran
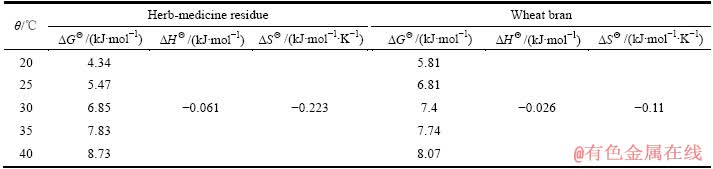
从表4可看出,中药渣和麦麸的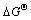 均为正值且随着温度的升高不断增大,说明反应非自发进行,需要外部提供能量,在本试验中,振荡可为其提供活化能;
均为正值且随着温度的升高不断增大,说明反应非自发进行,需要外部提供能量,在本试验中,振荡可为其提供活化能; 为负值说明反应放热,升温不利于吸附,与温度试验的结论一致;
为负值说明反应放热,升温不利于吸附,与温度试验的结论一致;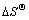 为负值说明反应过程混乱度降低,同样不利于自发反应。综上所述,在20~40 ℃下,中药渣和麦麸对Cu2+的吸附为非自发的放热反应。
为负值说明反应过程混乱度降低,同样不利于自发反应。综上所述,在20~40 ℃下,中药渣和麦麸对Cu2+的吸附为非自发的放热反应。
2.9 机理分析
2.9.1 Zeta电位分析
根据佟雪娇等[22]对Zeta电位的测定方法,测得中药渣和麦麸的Zeta电位随pH值的变化趋势,结果如图7所示。
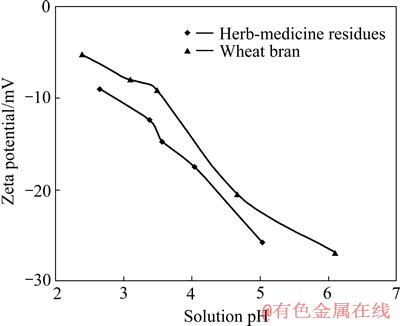
图7 中药渣和麦麸的Zeta电位
Fig. 7 Zeta potentials of herb-medicine residues and wheat bran
由图7可看出,中药渣和麦麸的Zeta电位为负值,且随着pH值的升高电负性变得更强,这说明中药渣和麦麸吸附Cu2+存在着物理吸附即静电吸附,且pH值越高静电吸附量越大。
2.9.2 FT-IR分析
对吸附前后的中药渣和麦麸进行红外光谱分析,结果分别如图8和9所示。
由图8可看出,中药渣的成分很复杂,在整个波段内有众多的吸收峰存在。根据文献[23]对吸附前的中药渣进行分析:3 402.52 cm-1附近的强峰为O—H的伸缩振动,3 008.57 cm-1附近的峰为芳环上C—H的伸缩振动,2 926.91 cm-1附近的强峰为饱和烃—CH2的反对称伸缩振动,2 855.35 cm-1附近的强峰为甲基中C—H的对称伸缩振动,1 744.64 cm-1附近的强峰为羧酸C=O的伸缩振动,1 649.64 cm-1附近的强峰为酰胺Ⅱ吸收带,1 600~1 450 cm-1处的峰为芳烃C=C骨架振动,1 237.86 cm-1附近的峰为酚羟基C—OH的伸缩振动,1 163.17 cm-1附近的峰为酯的C—O—C伸缩振动,1 102.98 cm-1附近的峰为醇羟基C—OH的伸缩振动,720.13 cm-1附近的峰为N—H的面外弯曲振动。
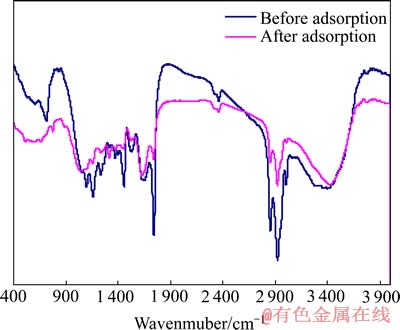
图8 中药渣吸附前后的红外光谱
Fig. 8 FT-IR spectra of herb-medicine residues before and after biosorption process
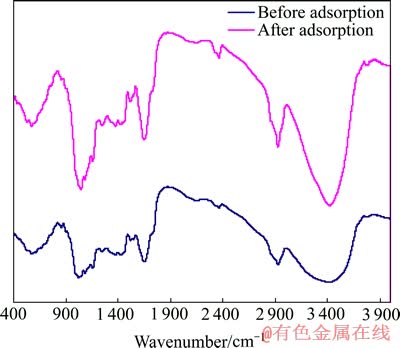
图9 麦麸吸附前后的红外光谱
Fig. 9 FT-IR spectra of wheat bran before and after biosorption process
中药渣吸附Cu2+后,其红外光谱图变化较大,主要有O—H的伸缩振动峰由3 402.52 cm-1移至3 425.75 cm-1,峰强变大;羧酸C=O的伸缩振动峰由1 744.64 cm-1移至1 742.94 cm-1,峰强变弱;酰胺Ⅱ吸收带由1 649.64 cm-1移至1 626.11 cm-1,峰强度变大;酚羟基C—OH的伸缩振动峰由1 237.86 cm-1移至1 239.62 cm-1,峰形稍变宽;酯的C—O—C伸缩振动峰由1 163.17 cm-1移至1 160.15 cm-1,峰强变弱;醇羟基C—OH的伸缩振动峰由1 102.98 cm-1移至1 052.17 cm-1,峰形变宽,峰强变弱;N—H的面外弯曲振动峰由720.13 cm-1移至780.05 cm-1,峰强变弱。由此说明,中药渣吸附Cu2+的主要官能团为羟基、羧基、酰胺基、酯基等。
由图9可看出,麦麸的成分较中药渣的简单得多,同样根据文献[23]对其进行分析可知:3 413.83 cm-1附近的强峰为O—H的伸缩振动,2 927.78 cm-1附近的强峰为饱和烃—CH2的反对称伸缩振动,1 648.96 cm-1附近的强峰为酰胺Ⅱ吸收带,1 600~1 450 cm-1处的峰为芳烃C=C骨架振动,1 082.28 cm-1、1 021.98 cm-1附近的峰为糖类C—OH的伸缩振动,892.89 cm-1附近的峰为有机硅Si—O—Si的对称伸缩振动。
麦麸吸附Cu2+后,透过率增大,且O—H的伸缩振动峰由3 413.83 cm-1移至3 422.46 cm-1,峰强变大;酰胺Ⅱ吸收带由1 648.96 cm-1移至1 640.73 cm-1,峰形、峰强变化不明显;糖类C—OH振动峰向高波数位移,且峰形变窄;有机硅Si—O—Si的对称伸缩振动峰由892.89 cm-1移至885.18 cm-1,峰强明显变小。由此可推断,麦麸吸附Cu2+的主要官能团为羟基、酰胺基、硅氧基等。
由于羟基、羧基、酰胺基等基团能够和Cu2+发生离子交换或配位络合反应,因此中药渣和麦麸对Cu2+的吸附也存在化学吸附。
综上所述,中药渣和麦麸吸附Cu2+时既存在物理吸附又存在化学吸附。
3 结论
1) 溶液pH值、初始重金属离子浓度、吸附剂投加量、吸附时间、吸附温度对中药渣和麦麸吸附Cu2+均有较大影响。
2) 中药渣和麦麸的吸附过程为非自发的放热反应,符合拟二级动力学模型;粒子内部扩散模型表明,二者对Cu2+的吸附要同时考虑膜扩散和粒子内部扩散。
3) 中药渣对Cu2+的吸附效果要优于麦麸的,二者的最大吸附量分别为14.03和7.34 mg/g,与其他吸附剂相比,在处理低pH值、高Cu2+浓度废水方面具有一定的竞争潜力。
4) 中药渣和麦麸吸附Cu2+为物理吸附和化学吸附共存,化学吸附的基团主要是羟基和酰胺基等。
REFERENCES
[1] 初 娜, 赵元艺, 张光第, 杨 慧. 江西省德兴铜矿矿区重金属元素的环境效应[J]. 地质学报, 2008, 82(4): 562-576.
CHU Na, ZHAO Yuan-yi, ZHANG Guang-di, YANG Hui. Environmental effect of heavy metal elements in Dexing Copper Mine, Jiangxi Province[J]. Acta Geologica Sinica, 2008, 82(4): 562-576.
[2] 杨 群, 宁 平, 陈芳媛, 赵天亮. 矿山酸性废水治理技术现状及进展[J]. 金属矿山, 2009(1): 131-134.
YANG Qun, NING Ping, CHEN Fang-yuan, ZHAO Tian-liang. Status and prospect of the treatment technology for acid mine drainage[J]. Metal Mine, 2009(1): 131-134.
[3] WITEK-KROWIAK A, SZAFRAN R G, MODELSKI S. Biosorption of heavy metals from aqueous solutions onto peanut shell as a low-cost biosorbent[J]. Desalination, 2011, 265: 126-134.
[4] PEHLIVAN E, ALTUN T. Biosorption of chromium(Ⅵ) ion from aqueous solution using walnut, hazelnut and almond shell[J]. Journal of Hazardous Materials, 2008, 155: 378-384.
[5] ARGUN M E, DURSUN S, OZDEMIR C, KARATAS M. Heavy metal adsorption by modified oak sawdust: Thermodynamics and kinetics[J]. Journal of Hazardous Materials, 2007, 141: 77-85.
[6]  M. Adsorption of heavy metals from electroplating wastewater by wood sawdust[J]. Bioresource Technology, 2007, 98: 402-409.
M. Adsorption of heavy metals from electroplating wastewater by wood sawdust[J]. Bioresource Technology, 2007, 98: 402-409.
[7] GUNDOGDU A, OZDES D, DURAN C, BULUT V N, SOYLAK M, SENTURK H B. Biosorption of Pb(Ⅱ) ions from aqueous solution by pine bark (Pinus brutia Ten.)[J]. Chemical Engineering Journal, 2009, 153: 62-69.
[8] SARIN V, PANT K K. Removal of chromium from industrial waste by using eucalyptus bark[J]. Bioresource Technology, 2006, 97: 15-20.
[9] BANSAL M, GARG U, SINGH D, GARG V K. Removal of Cr(VI) from aqueous solutions using pre-consumer processing agricultural waste: A case study of rice husk[J]. Journal of Hazardous Materials, 2009, 162: 312-320.
[10] KRISHNANI K K, MENG Xiao-guang, CHRISTODOULATOS C, BODDU V M. Biosorption mechanism of nine different heavy metals onto biomatrix from rice husk[J]. Journal of Hazardous Materials, 2008, 153: 1222-1234.
[11] LIU C F, SUN R C, QIN M H, ZHANG A P, REN J L, YE J, LUO W, CAO Z N. Succinoylation of sugarcane bagasse under ultrasound irradiation[J]. Bioresource Technology, 2008, 99(5): 1465-1473.
[12] 姜 玉, 庞 浩, 廖 兵. 甘蔗渣吸附剂的制备及其对Pb2+、Cu2+、Cr3+的吸附动力学研究[J]. 中山大学学报: 自然科学版, 2008, 47(6): 32-37.
JIANG Yu, PANG Hao, LIAO Bing. Preparation of bagasse adsorbents and investigation of their adsorption kinetic of Pb(Ⅱ), Cu(Ⅱ) and Cr(Ⅲ)[J]. Acta Scientiarum Naturalium Universitatis Sunyatseni, 2008, 47(6): 32-37.
[13] 韦平英, 魏东林, 莫德清. 板蓝根药渣对低浓度含铅废水的吸附特性研究[J]. 离子交换与吸附, 2003, 19(4): 351-356.
WEI Ping-ying, WEI Dong-lin, MO De-qing. Biosorption of lead by Isatis indigotica Fort draff[J]. Ion Exchange and Adsorption, 2003, 19(4): 351-356.
[14] WANG Xue-song, LI Zhi-zhong, SUN Cheng. A comparative study of removal of Cu(Ⅱ) from aqueous solutions by locally low-cost materials: Marine macroalgae and agricultural by-products[J]. Desalination, 2009, 235: 146-159.
[15] 赵艳春, 张文德. BCO测定铜方法的改进[J]. 中国卫生检验杂志, 1995(1): 47-48.
ZHAO Yan-chun, ZHANG Wen-de. Improvement of BCO determining copper[J]. Chinese Journal of Health Laboratory Technology, 1995(1): 47-48.
[16] ZHANG Yun-song, LIU Wei-guo, ZHANG Li, WANG Meng, ZHAO Mao-jun. Application of bifunctional accharomyces cerevisiae to remove lead(Ⅰ) and cadmium(Ⅱ) in aqueous solution[J]. Applied Surface Science, 2011, 257: 9809-9816.
[17] 郭学益, 公琪琪, 梁 莎, 田庆华, 肖彩梅. 改性柿子生物吸附剂对铜和铅的吸附性能[J]. 中国有色金属学报, 2012, 22(2): 599-603.
GUO Xue-yi, GONG Qi-qi, LIANG Sha, TIAN Qing-hua, XIAO Cai-mei. Adsorption properties of modified persimmon biosorbent on Cu2+ and Pb2+[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(2): 599-603.
[18] 熊百炼. 甘蔗渣吸附废水中Cd2+和Cr3+的研究[D]. 重庆: 西南大学, 2009: 27.
XIONG Bai-lian. Investigation of the adsorption characteristic of the bagasses to Cd2+ and Cr3+ in wastewater[D]. Chongqing: Southwest University, 2009: 27.
[19] CHOONG J. Removal of copper ion using rice hulls[J]. Journal of Industrial and Engineering Chemistry, 2011, 17: 517-520.
[20] ABBAS H S, BALASIM A A, JENAN A A. Removal of lead copper chromium and cobalt ions onto granular activated carbon in batch and fixed-bed adsorbers[J]. Chemical Engineering Journal, 2009, 155: 647-653.
[21] 郭学益, 梁 莎, 肖彩梅, 田庆华. MgCl2改性柑橘皮对水溶液中重金属离子的吸附性能[J]. 中国有色金属学报, 2011, 21(9): 2270-2276.
GUO Xue-yi, LIANG Sha, XIAO Cai-mei, TIAN Qing-hua. Adsorption of heavy metal ions from aqueous solutions by MgCl2 modified orange peel[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(9): 2270-2276.
[22] 佟雪娇, 李九玉, 袁金华, 徐仁扣, 周立祥. 稻草炭对溶液中Cu(Ⅱ)的吸附作用[J]. 环境化学, 2012, 31(1): 64-68.
TONG Xue-jiao, LI Jiu-yu, YUAN Jin-hua, XU Ren-kou, ZHOU Li-xiang. Adsorption of Cu(Ⅱ) on rice straw char from acidic aqueous solutions[J]. Environmental Chemistry, 2012, 31(1): 64-68.
[23] 翁诗甫. 傅里叶变换红外光谱分析[M]. 2版. 北京: 化学工业出版社, 2010: 291-358.
WENG Shi-fu. Fourier transform infrared spectroscopy analysis[M]. 2nd ed. Beijing: Chemical Industry Press, 2010: 291-358.
(编辑 龙怀中)
基金项目:环保部环保公益性行业科研专项资助项目(201209013)
收稿日期:2012-04-28;修订日期:2012-09-20
通信作者:陈月芳,副教授,博士;电话:18010078346;E-mail: yuefangchen@vip.sina.com