三价铬还原电沉积机理
邓姝皓1,龚竹青2,易丹青1, 苏玉长1
(1.中南大学 材料科学与工程学院,湖南 长沙,410083;
2.中南大学 冶金科学与工程学院,湖南 长沙,410083)
摘要: 采用循环伏安法、极化曲线、恒电流阶跃、交流阻抗等电化学方法研究了Cr3+在氯化物/N,N-二甲基甲酰胺(DMF)体系中的阴极还原机理。研究结果表明:Cr3+阴极还原分两步进行,其中第1步为不可逆过程,第2步为准可逆过程;反应无前置转化过程;Cr3+还原的极化曲线符合Tafel方程;Cr3+还原受电化学控制;Cr3+在还原过程中,电活性中间产物在电极表面吸附,随溶液浓度增大,Cr3+的扩散系数减小,但电活性中间产物在电极表面的饱和吸附量却随之增大。
关键词: 三价铬; 电化学; 还原机理
中图分类号:O646.54 文献标识码:A 文章编号: 1672-7207(2005)02-0213-06
Electrochemical Mechanism of Trivalent Chromium Electrodeposition
Deng Shu-hao1, Gong Zhu-qing2, Yi Dan-qing1, Su Yu-chang1
(1.School of Materials Science and Engineering, Central South University, Changsha 410083, China;
2.School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: The reduction mechanism of Cr3+ in chloride/ N, N-dimethylformamide (DMF) solution was studied using several kinds of electrochemical methods, such as cyclic voltammetry, steady polarization curve, chronopotentiometry, and alternating current impedance. The results show that Cr3+ gets three electrons in two steps, the first step is nonreversible, while the second step is quasi-reversible, and the trivalent Cr3+ does not undergo chemical transformation reaction before its reduction in the cathode. The steady polarization curve of Cr3+ reduction is fit to Tafel equation, and the calculated values of active energy indicate the reduction process is determined by electrochemical step. The middle product, which is electrochemically active and adsorptive, is formed in the first step. The values of diffusion coefficient of Cr3+ and saturated adsorption of middle product calculated from chronocoulometry method reveal that the greater the concentration of Cr3+, the smaller diffusion coefficient, and the more saturated adsorption of the middle product.
Key words: trivalent chromium; electrochemistry; reduction mechanism
三价铬镀液中沉积铬的工艺具有工艺简单和环保等方面的优点而受到广泛的重视,因此,研究三价铬电镀的机理具有十分重要的现实意义和理论意义。目前,人们对三价铬沉积的机理进行了许多研究,如采用循环伏安法确定铬沉积时的电子传递系数和反应步骤[1-5],测定铬沉积时的Tafel曲线斜率[6,7],用极谱法等确定Cr3+的扩散系数和反应速[CM(22] 度常数等[8]。但是,由于从三价铬镀液中沉积铬的体系各不相同,对其机理的研究很难获得一致的结果。
在此,作者采用循环伏安法、极化曲线、恒电流阶跃、交流阻抗等多种电化学方法研究Cr3+在氯化物/N,N-二甲基甲酰胺(DMF)体系中铬的阴极沉积机理,确定所研究体系中铬沉积的阴极反应历程。
1 实验方法
实验采用三电极体系,研究电极为铜电极(直径为1 mm),辅助电极为大面积的铂片,参比电极为饱和甘汞电极。采用带有Luggin毛细管的盐桥以消除不同溶液间的液体接界电位。实验前,工作电极用金相砂纸抛光打磨,用酒精、丙酮清洗,用稀盐酸活化,再用蒸馏水洗涤。实验前,往溶液中通15 min氮气以去除溶解的氧。电解液均采用分析纯试剂,用蒸馏水配置,溶液pH值为1.0~2.0,温度为15~65 ℃。电解液成分如表1所示。
采用循环伏安法、极化曲线、电流阶跃法、交流阻抗法等多种电化学方法研究Cr3+的阴极还原机理;并用计时电量法计算电活性中间产物的理论吸附量。以上测试均采用电化学工作站CHI660A进行(所测电位均是相对于饱和甘汞电极电位而言的)。
表 1 电解液成分
Table 1 Compositions of electrolyte

2 结果与讨论
2.1 Cr3+还原的循环伏安曲线
Cr3+在铜电极上还原的循环伏安曲线如图1所示。循环伏安曲线上分别在-1.2 V(峰A)和-1.5 V(峰B)附近出现了2个峰。当扫描速度较低,并且在峰A后进行回扫时,没有看到响应的氧化峰,说明峰A对应的反应是不可逆的;但是,当在峰B后进行回扫,发现峰B在扫速较小时,有阳极峰对应,但在扫速较高时没有对应的氧化峰,说明此反应为准可逆的。由此可见Cr3+的还原反应分2步进行。
2.1.1 Cr3+还原的第1个峰
在扫描速度v为25~200 mV/s时,在Cr3+还原反应循环伏安曲线上的第1个峰如图2所示。相应的扫描速度的平方根 与峰电流密度Jp1/
与峰电流密度Jp1/ 的关系如图3所示。从图3可见,Jp1/
的关系如图3所示。从图3可见,Jp1/ 与
与 呈水平直线。由于循环伏安曲线在回扫时无论速度高低均无对应的阳极峰,并且峰电位不随扫描速度增加而向阴极方向移动,说明Cr3+还原的第1个峰所对应的第1个还原过程受电化学控制,是不可逆的,此过程为:
呈水平直线。由于循环伏安曲线在回扫时无论速度高低均无对应的阳极峰,并且峰电位不随扫描速度增加而向阴极方向移动,说明Cr3+还原的第1个峰所对应的第1个还原过程受电化学控制,是不可逆的,此过程为:
Cr3++e→Cr2+。
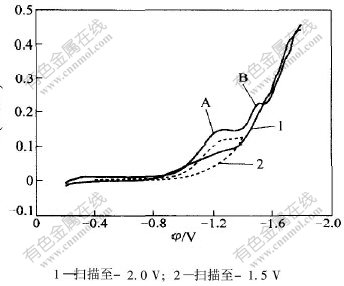
图 1 Cr3+还原的循环伏安曲线
Fig. 1 Cyclic voltammetry curves for Cr3+ cathodic reduction
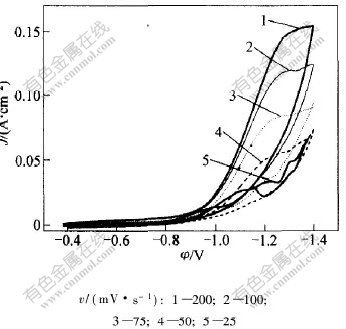
图 2 不同扫描Cr3+还原的循还伏安曲线上的第1个峰
Fig. 2 The first peak of cyclic voltammetry curves for Cr3+cathodic reduction at different scan rates
2.1.2 Cr3+还原的第2个峰
当扫描电位远负于Cr3+/Cr2+电位时,1个新的还原波出现在-1.4 V附近,如图4所示。还原峰A和峰B的高度比约为1∶3。这说明峰B的电流为峰A的3倍。可以推出峰B对应的反应为:
Cr2++2e→Cr。
由于峰B只在低速扫描时有相应的氧化峰对应,峰电位随扫描速度增加不断向阴极方向移动, Jp2/ 与
与 的关系如图5所示,可见Jp2/
的关系如图5所示,可见Jp2/ 与
与 近似呈水平直线关系。这说明Cr2+还原为Cr的过程是准可逆的过程。
近似呈水平直线关系。这说明Cr2+还原为Cr的过程是准可逆的过程。
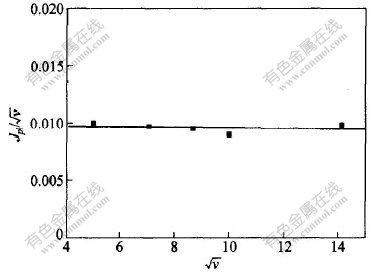
图 3 Jp1/ 与扫描速度的平方根
与扫描速度的平方根 的关系
的关系
Fig. 3 Relationship between Jp1/ and
and 
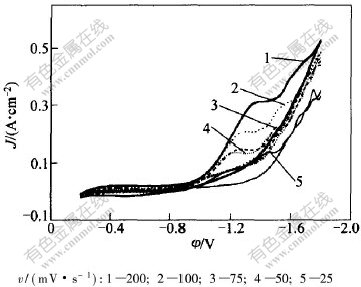
图 4 不同扫描速度下Cr3+还原的循环伏安曲线上第2个峰
Fig. 4 The second peak of cyclic voltammetry curves for Cr3+cathodic reduction at different scan rates
2.2 Cr3+还原的极化曲线
采用慢扫描(扫描速度为1 mV/s)测量铜电极上Cr3+还原极化曲线,如图6所示。从图6可以看出,曲线大体上可以分为3段:
-0.8~-1.0 V为第1段,在此区间,电流上升平缓,铜电极上无铬沉积。反应方程式为Cr3++e→Cr2+。
在-1.2~-1.8 V区间,电流随电位负移迅速上升,观察铜电极可以看到有蓝亮色膜,说明铬沉积反应出现,此段区间还出现了氢的还原,这与文献[9]报道的结果一致。随沉积电位负移而显现为金属铬。
在恒电位-1.1 V下长时间沉积,铜电极上无任何金属膜出现。但若在铬-镍-铁合金镀液中-1.1 V下长时间沉积,则可以在绝缘胶纸和基体金属上得到黑色的质量差而疏松的镀层,这是由于发生反应Cr3++e→Cr2+后,Cr2+化学催化还原Ni2+,Fe2+生成镍,铁的化学镀层和镍-铁合金镀层所造成的。电位负于-1.8 V后,氢析出剧烈。
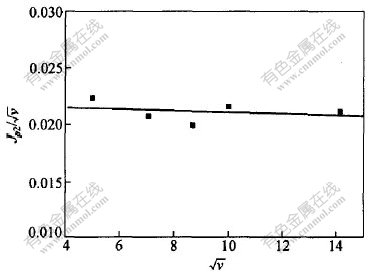
图 5 Jp2/ 与扫描速度的平方根
与扫描速度的平方根 的关系
的关系
Fig. 5 Relationship between Jp2/ and
and 
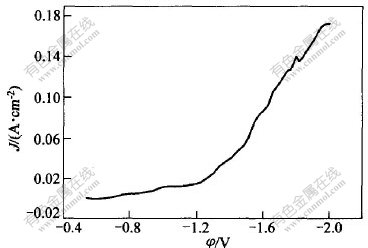
图 6 Cr3+还原的电位与电流密度的关系
Fig. 6 Relationship between current density and potential for Cr3+ cathodic reduction
极化曲线在前2个电位范围都符合Tafel方程,通过计算,电位在-0.8~-1.0 V时的Tafel斜率b=0.182,nα1=0.33(t=30 ℃),电位在-1.2~-1.8 V时的Tafel斜率b=0.41,nα2=0.148(t=30 ℃)(其中,n为电子数目,α1和α2分别为第1步和第2步传递系数)。
2.3 控制步骤性质的确定
2.3.1 控制步骤性质的测定
当电极反应受扩散控制时,电极反应的活化能较低;而电化学反应的温度系数很大,电极反应的活化能也很大。然而,如果存在化学转化的前置反应,因为其类似于扩散,所以无法用活化能大小直接判断。为确定是否存在前置反应,首先进行了不同电流密度下的恒电流阶跃实验。
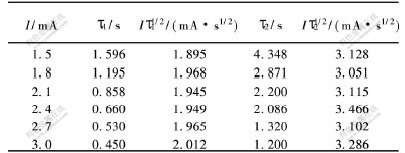
L.BONOU等[10]对铜在玻璃碳电极电位-时间暂态曲线进行了研究,认为若Iτ1/2基本保持恒定(其中:I为电流,τ为迁移时间),表明电极反应为无前置转化反应。根据文献[3]报道,采用循环伏安曲线上大于第1个峰的电流的1/10作为恒电流阶跃的起始电流,实验结果如表2所示。从表2可以看到,随阶跃电流增大,τ值依次减小。
由于Iτ1/22和Iτ1/21对I作图均呈水平直线,说明Iτ1/22与电流无关,Cr3+还原为Cr2+及Cr2+还原为Cr的反应没有前置反应(表面转化步骤)发生。
表 2 Cr3+还原的电流与迁移时间的关系
Table 2 Relationship between current and transition time for Cr3+ cathodic reduction
2.3.2 Cr3+还原活化能
在不同温度下铜电极上Cr3+的阴极极化曲线如图7所示。从图7可见,随镀液温度升高,铬在铜电极上电沉积的阴极极化不断降低。将图7中电流密度J的对数对温度的倒数 作图,发现lgJ与
作图,发现lgJ与 存在线性关系。
存在线性关系。

图 7 不同温度下Cr3+阴极极化曲线
Fig. 7 Cathodic polarization curves for Cr3+at different temperatures
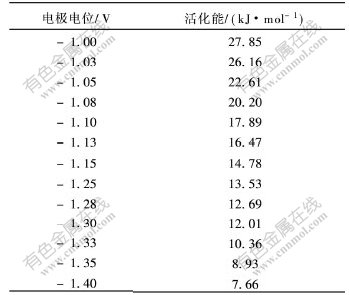
由直线的斜率可求出Cr3+在铜电极上沉积时不同电位下的活化能,结果如表3所示。通过对不同温度下的交换电流密度的对数与温度的倒数作图,还可以计算其平衡电位下的活化能[11]。
从表3可知,电位在-1.0~-1.10 V时,活化能为18~28 kJ/mol;电位在-1.10~-2.05 V时,活化能小于18 kJ/mol;其平衡电位下的活化能为42.4 kJ/mol,从Cr3+溶液中沉积铬的表观活化能都比较大,说明Cr3+在DMF/水溶液中还原为单质铬的过程主要是受电化学反应控制。
表 3 不同沉积电位下的活化能
Table 3 Active energies for Cr3+ cathodic reduction at different potentials
2.4 Cr3+还原的交流阻抗
Cr3+从DMF水溶液中不同电极电位下沉积铬的交流阻抗如图8所示。可以看出,由交流阻抗所得到的是一系列由前、后半径不同的2个圆弧组成的图形,其圆心落在与实轴成α角的轴上。电位为-0.9~-1.1 V时(见图8(a)),阻抗显示为前小后大的2个圆弧,并且随着电位负移,前面圆弧的半径逐渐增大,后面圆弧的半径则相应减小。此时,铜电极上无任何铬沉积。因此,可以断定,此时的电极反应为电化学控制的氢离子放电反应[12-14] 以及Cr3+转化为Cr2+的反应。阻抗谱中,后面诱导圆弧的出现表明铬沉积过程中出现了电活性中间产物吸附在电极表面,由于溶液中无任何有机添加剂存在,可认为是Cr3+还原为Cr2+吸附在电极表面。电位为-1.2 V时(见图8(b)),电极上开始有铬沉积,此时阻抗图显示的是前大后小的2个圆弧,随着电极电位的负移,前面圆弧的半径开始减小,电位在-1.6~-1.7 V之间时(见图8(c)),在2个圆弧之间还出现了1个线圈(低频时),随电位负移,此线圈逐渐消失,这是氢离子放电引起的,它对铬沉积的电极过程起着很大的作用。当电极电位负移至-1.8 V时(见图8(d)),后面的吸附圆弧开始向第4象限延伸,并与前面的大圆弧逐渐融合;同时,前面圆弧的半径也随电位负移而减小。说明此电极上的反应是以氢离子放电为主的电化学反应,铬沉积反应居于次要地位。
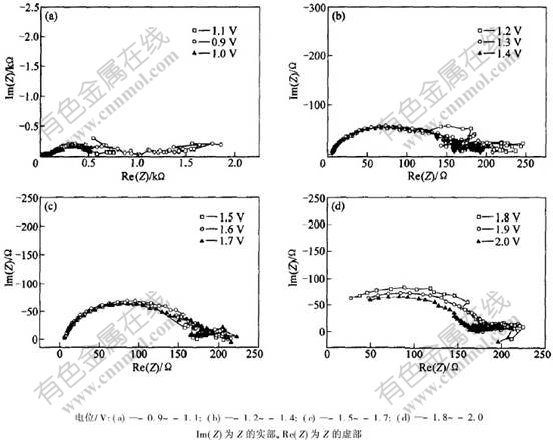
图 8 Cr3+还原的交流阻抗图
Fig. 8 Alternating current impedance for Cr3+ cathodic reduction
2.5 电活性中间体Cr2+的吸附
为了测定Cr3+还原为Cr2+时电极吸附量的大小,选用计时库仑法[15]测定了在相同温度下不同Cr3+浓度还原时电活性中间体Cr2+在电极表面的饱和吸附量Γ∞以及Cr3+扩散系数。测定结果如表4所示。
从表4可以看出,扩散系数随Cr3+浓度的增大而减小。由于Cr3+在溶液中容易与DMF和水形成配和物,随着Cr3+浓度增大,单位体积内可以运动的空间减小,同时,溶液粘度也增大,故Cr3+的扩散系数减小。
表 4 Cr3+浓度与电活性物质饱和吸附量Γ∞及扩散系数DCr3+的关系
Table 4 Relationship between concentration of Cr3+, electroactive saturated adsorption and diffusion coefficient

3 结 论
a. Cr3+在DMF/水溶液中还原分2步进行:第1步为不可逆反应,第2步为准可逆反应。
b. Cr3+在DMF/水溶液中还原为Cr2+符合Tafel公式,受电化学控制。
c. Cr3+在DMF/水溶液中还原无前置转化反应,其过程由电化学控制。
d. Cr3+在DMF/水溶液中还原的过程中有电活性中间产物在电极表面吸附,Cr3+的扩散系数随浓度增大而减小,但电活性中间产物的饱和吸附量却随之增大。
参考文献:
[1]HSIEH A K, EE Y H, CHEN K N. Electrochemistry of Chromium Deposition from Thiocyanato Trivalent System[J]. Metal Finishing, 1993, 91(4): 53-57.
[2]KVETA M O. Electroreduction of Chromium(III) Chloride Complexes at Mercury in Dimethylformamide Solutions[J]. Journal of Electroanalytical Chemistry, 1991, 308(1-2): 203-211.
[3]SZYNKARCZUK J, DRELA I, KUBICKI J. Electrochemical Behaviour of Chromium(Ⅲ) in the Presence of Formic Acid[J]. Electrochimica Acta, 1989, 34(3): 399-403.
[4]何湘柱,龚竹青,蒋汉瀛.三价铬离子水溶液电沉积非晶态铬的电化学[J].中国有色金属学报,2000,10(1):95-99.
HE Xiang-zhu, GONG Zhu-qing, JIANG Han-ying. Electrodeposition of Amorphous Chromium from Cr(Ⅲ) Aqeous Solution[J]. The Chinese Journal of Nonferrous Metals, 2000, 10(1): 95-99.
[5]MARTNEZ A M, CASTRILLEJO Y, BORRESEN B, et al. Chemical and Electrochemical Behaviour of Chromium in Molten Chlorides[J]. Journal of Electroanal Chem, 2000, 493(1): 1-14.
[6]SONG Y B, CHIN D T. Current Efficiency and Polarization Behavior of Trivalent Chromium Electrodeposition Process[J]. Electrochimica Acta, 2002, 48(4): 349-356.
[7]DRELA I, SZYNKARCZUK J, KUBICKI J. Electroduction of Chromium-acetate Complex to Metallic Chromium on the Copper Electrode[J]. Electrochimica Acta, 1988, 33(4): 589-592.
[8]BIMAGHRA I, CROUSIER J. Electrodeposition of Copper from Sulphate Solution-influence of the Cations[J]. Materials Chemistry and Physics, 1989, 21(2): 109-122.
[9]何湘柱.非晶态Fe-Ni-Cr合金电沉积工艺及理论研究[D]. 长沙:中南工业大学冶金科学与工程学院, 1999.
HE Xiang-zhu. Studies on Technology and Basic Mechanism of Electrodeposition Amorphous Fe-Ni-Cr Alloy[D]. Changsha: School of Metallurgical Science and Engineering, Central South University of Technology, 1999.
[10]BONOU L, EYRAUD M, CROUSIER J. Nucleation and Growth of Copper on Glass Carbon and Steel[J]. J Appl Electrochem, 1994, 24(9): 906-910.
[11]舒余德,陈白珍.冶金电化学研究方法[M]. 长沙:中南工业大学出版社,1990.
SHU Yu-de, CHEN Bai-zhen. Methods of Metallurgical Electrochemistry[M]. Changsha: Central South University of Technology Press, 1990.
[12]SONG Y B, CHIN D T. Pulse Plating of Hard Chromium from Trivalent Baths[J]. Plating & Surface Finishing, 2000, 87(9): 80-87.
[13]SONG Y B. Study of Pulse Plating and Reaction Mechanism of Trivalent Chromium Deposition Process[D]. Canada: Department of Chemistry, Clarkson University, 2000.
[14]WANG Feng, WATANABE T. Preparation and Characterization of the Electrodeposited Fe-Cr Alloy Film[J]. Materials Science and Engineering A, 2003, 349(1-2): 183-190.
[15]SHIN H, PARK M, KIM A R, et al. Electrochemical Measurements of Cu2+ Ions Adsorbed on an Electrochemically Oxidized Glassy Carbon Electrode[J]. Journal of Electroanalytical Chemistry, 2003, 547(2): 143-149.
收稿日期:2004-10-20
作者简介:邓姝皓(1974-),女,湖南衡阳人,讲师,博士,从事材料、电化学研究
论文联系人: 邓姝皓,女,讲师,博士;电话:0731-8836320(O)