Thermodynamic analysis of interactions between Ti-Al alloys and oxide ceramics
来源期刊:中国有色金属学报(英文版)2012年第4期
论文作者:崔仁杰 唐晓霞 高明 张虎 宫声凯
文章页码:887 - 894
关键词:Ti合金;热力学;氧化物陶瓷;界面反应
Key words:Ti alloys; thermodynamics; oxide ceramics; interaction
摘 要:研究CaO、MgO、Al2O3和Y2O3与熔融Ti及Ti合金之间的界面反应热力学。根据氧化物在Ti合金熔体中的溶解反应机制建立热力学模型,通过热力学分析预测各氧化物相对于Ti-Al(0≤x(Al)≤0.5)合金的热力学稳定性。根据氧化物坩埚熔炼实验结果来研究氧化物与Ti-xAl合金熔体的界面反应,对熔炼效果进行定量分析,发现随着合金中Al含量的提高和熔体温度的降低,合金中杂质元素含量成比例的减少,这与氧化物稳定性计算结果一致。
Abstract: The thermodynamics of interactions between various oxides (CaO, MgO, Al2O3 and Y2O3) and molten Ti and Ti alloys was investigated. The dissolution mechanism of oxides in molten Ti alloys was provided and the stability of oxides in molten Ti alloys was investigated and predicted by thermodynamic analysis. Interactions between oxides and Ti-Al melts were studied by oxide crucible melting experiments. By quantitative analysis, it is indicated that impurity contents in alloys are proportionally decreased with increasing the Al content in alloys and decreasing the melt temperature, which is in agreement with the results of the predicting thermodynamic stability.

![]()

Trans. Nonferrous Met. Soc. China 22(2012) 887-894
CUI Ren-jie, TANG Xiao-xia, GAO Ming, ZHANG Hu, GONG Sheng-kai
Key Laboratory of Aerospace Materials and Performance (Ministry of Education),
School of Materials Science and Engineering, Beihang University, Beijing 100191, China
Received 18 May 2011; accepted 10 October 2011
Abstract: The thermodynamics of interactions between various oxides (CaO, MgO, Al2O3 and Y2O3) and molten Ti and Ti alloys was investigated. The dissolution mechanism of oxides in molten Ti alloys was provided and the stability of oxides in molten Ti alloys was investigated and predicted by thermodynamic analysis. Interactions between oxides and Ti-Al melts were studied by oxide crucible melting experiments. By quantitative analysis, it is indicated that impurity contents in alloys are proportionally decreased with increasing the Al content in alloys and decreasing the melt temperature, which is in agreement with the results of the predicting thermodynamic stability.
Key words: Ti alloys; thermodynamics; oxide ceramics; interaction
1 Introduction
Ti alloy is an important structural material, due to its high specific strength, good biocompatibility, high corrosion resistance and many other advantages. At the present stage, Ti alloy is a promising metallic material and its production is widely used in emerging markets, such as aerospace, military, petroleumical industry, textile, biomedical devices, automotive industry, and sports [1,2]. TiAl alloys have attracted attention as potential candidates for high-temperature structural applications in the aerospace and military [3]. However, the main factors to limit the manufacture of mass market Ti alloy components are the high reactivity of Ti alloys with crucible/mould refractory materials at high temperatures. When the interactions between the alloys and crucible/mould materials occur, the microstructure and properties of the castings will be affected. Therefore, it is necessary to investigate the interactions between refractory materials and Ti alloy melts and select suitable refractory materials as crucibles or moulds for melting or casting the alloy.
At present, many researchers have investigated interactions between Ti alloy melts and refractory materials, including borides, carbides, sulfide, nitride and oxide [4,5]. The results show that the oxide family of materials receives the most attention, due to its low cost, high melting point and high refractoriness. Moreover, the Gibbs free energy diagram of oxides can also be useful for comparison of the relative stability of oxides. However, SAHA et al [2] experimentally found that the stability of various oxides against Ti melts is not consistent with the free energy of formation of these oxides, indicating that the standard free energy diagram does not provide a correct picture of the reactivity of oxides with liquid metal. Researches so far indicate that the solution effects of oxides in liquid metal need to be taken into consideration to obtain a more realistic picture. Many researchers [6-10] have studied the thermo- dynamic stability of oxides against Ti alloy melts, but mainly focus on experimental aspects, whose cost is high while accuracy is low. Therefore, it is instructive to theoretically predict the thermodynamic stability of oxides and investigate interactions between Ti alloy melts and oxides.
In this study, the dissolution mechanism of oxides in molten Ti alloys was provided and the stability of various oxides were investigated and predicted by thermodynamic analysis. Moreover, melting experiments of Ti-xAl (x=0, 25%, 43%, 47%, mole fraction) alloys were performed to study the oxide-melt interaction. The melting effect was quantitatively analyzed and compared with the oxide stability.
2 Experimental
2.1 Model
In TiAl melts, the chemical reactivity of the melts depends on the chemical activities of Ti and Al elements in the melts. According to the basic thermodynamics of alloy melts and the previous theoretical and experimental research on TiA1 melts [11-13], the activities of Ti and Al in TiAl melts can be quantitatively calculated, which provides the basis for the study of the interaction mechanism between TiA1 melts and the oxide materials.
In binary i-j systems, the relationship between the partial molar excess free energy (![]() ) and the activity coefficient (γi) of component i is expressed as follows:
) and the activity coefficient (γi) of component i is expressed as follows:
![]() (1)
(1)
and the molar excess free energy is defined as:
![]() (2)
(2)
where xi is the mole fraction of component i; ![]() is the partial molar excess free energy of component i;
is the partial molar excess free energy of component i; ![]() is the excess free energy of binary i-j system and can be defined as:
is the excess free energy of binary i-j system and can be defined as:
![]() (3)
(3)
in which ΔHij is the enthalpy of formation during the alloying process between component i and j; ![]() is the excess entropy of components i and j. A connection between ΔHij and
is the excess entropy of components i and j. A connection between ΔHij and ![]() can be written as [14]:
can be written as [14]:
![]() (4)
(4)
![]() (5)
(5)
where
![]()
Tmi and Tmj are melting points of the components i and j, respectively. The formation enthalpy ΔHij can be calculated from Miedema model [15]. The Miedema model of formation of binary alloys has been widely applied to forecasting the formation enthalpy of binary metal solutions. Many researchers have studied the chemical activity coefficient of the components in binary systems based on this model, and the calculated results are identical to the experimental data [11,12]. According to the Miedema model, the formation enthalpy of binary i-j solutions ΔHij can be written as:
![]()
![]() (6)
(6)
where,
![]()
![]() , xi and xj are the mole
, xi and xj are the mole
fraction of i and j, respectively; Vi and Vj are the mole volumes of i and j, respectively; (nws)i and (nws)j are the electronic density of i and j, respectively; fi, fj are electronic charges of i and j, respectively; p, q, r, α are the experimental constants, where q/p=9.4, α=0.94, p=12.3 for binary TiAl melts [12].
Using Eqs. (1)-(6), the calculation model for chemical activity coefficient (γi) of the component i in binary i-j systems is expressed as:

![]() (7)
(7)
2.2 Melting experiment
In this work, TiAl alloys with nominal compositions of Ti-xAl (x=0, 25%, 43%, 47%, mole fraction) were produced from pure elements, titanium sponge (mass fraction, 99.76%) and high purity Al ingot (99.99%). Special care was taken to clean the raw materials, using acetone solution in an ultrasonic bath. After cleaning, they were dried at 150 °C for 120 min. The master alloy was prepared as a button of 1000 g by arc melting in a high purity argon atmosphere using a non–consumable tungsten electrode. The button was melted and turned five times to promote homogeneity. In order to prepare samples that would fit into the crucibles, the arc–melted button was cut to give master alloy samples with dimensions of 15 mm (diameter)×20 mm (height) using electrical discharge machining (EDM). Before the melting experiments, these samples were ground, cleaned and dried using the same procedure as described for the raw materials.
Melting was performed using a modified Bridgman furnace equipped with an yttria crucible (20 mm (out diameter)×15 mm (inner diameter)×20 mm (inner height), purity 99.9%), as shown in Fig. 1. Before the heating cycle, the furnace chamber was evacuated down to 6×10-3 Pa. After switching on the furnace power and heating up to 400 °C (keep evacuating), the furnace was backfilled with high purity argon (φ(O2)<2×10-6, φ(N2)<5×10-6, φ(H2)<1×10-6, φ(H2O)<1×10-6, φ(CH4)<1×10-6) up to 0.05 MPa, in order to reduce the oxygen and hydrosphere contents to a minimum level and avoid the evaporation of alloy components. After 40 min since the start of the heating cycle, the temperature reached 1400 °C. Afterwards, the heating rate was increased in order to reach the desired temperature as fast as possible. After reaching the temperature measured and controlled by a thermocouple A (WRe5–WRe26, Fig. 1) combined with an infrared pyrometer, the melt inside the crucible was stabilized at this temperature for 600 s in the furnace and then the furnace power was switched off. Finally, the crucible containing the cast ingot was removed from the furnace at a temperature of about 50 °C.
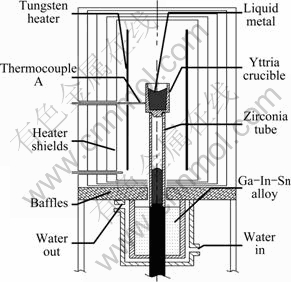
Fig. 1 Schematic diagram of modified Bridgman furnace equipped with yttria crucible
2.3 Characterization
The total oxygen contents of all ingots that were exposed to the yttria crucibles were measured by the inert gas infrared–thermal conductivity technique (IGI, LECO TC-436), with a measuring accuracy of ±1×10-6. For each ingot, two 0.8-1.2 g cylindrical bars parallel to the longitudinal axis of the ingot were taken at a distance of 3 mm from its surface. After being ground to a final mesh size of 120#, cleaned and dried using the same procedure as described for the raw materials, these bars were used for chemical analysis.
3 Results and discussion
3.1 Chemical activity of components in binary TiAl alloys
In order to study the thermodynamic stability of oxides against TiAl melts, the activity coefficients of the components Ti and Al as a function of temperature need to be known. The basic properties of some chemical elements and parameters used for calculations are listed in Table 1. By Eq. (7), the activity coefficients of Ti and Al in TiAl melts with different Al contents are calculated in a temperature range of 1800-2300 K, respectively. The activity coefficient—temperature—composition relationship for Ti and Al is shown in Fig. 2. The results presented in Fig. 2 show that the activity coefficient of the components is less than unity and the activity coefficient of Ti or Al increases with increasing its content, indicating that binary TiAl melts take on a negative deviation from the ideal solution. With increasing temperatures, each component’s activity coefficient increases, and the activity coefficient— temperature relationship can be expressed as:
ln γi =A+B/T (8)
where A and B are constants, whose values can be obtained by regression analysis of the data presented in Fig. 2.
Tables 2 and 3 show the estimated coefficients A and B for the activity coefficient—temperature relationships in TiAl alloys (0≤x(Al)≤0.5) in a temperature range between 1800 and 2300 K. Thus, as a first approximation the activity coefficient—temperature relationship of the components in TiAl alloys is formulated.
Table 1 Basic properties and parameters of Ti and Al [12] (electronic charge, electronic density, mole volume and experimental constants)


Fig. 2 Activity coefficient—temperature—composition relationship for Ti (a) and Al (b) in binary TiAl alloys
Table 2 Activity coefficient—temperature relationship for binary TiAl (0≤ x(Al)≤0.5) alloys (ln γ(Ti)=A+B/T)

Table 3 Activity coefficient—temperature relationship for binary TiAl (0≤ x(Al)≤0.5) alloys (ln γ(Al)=A+B/T)

3.2 Thermodynamics of interactions between oxide ceramics and Ti alloy melts
The interaction between oxide ceramics and TiAl melts is a complex physical and chemical process, and many researchers have studied the mechanisms of the interaction by experiments. Researches indicated that the most probable reason of alloy contamination is the dissolution of oxides by molten Ti alloys [9,16,17]. One possible mode of the interaction between Ti alloy melts and oxides (MxOy) is the dissolution of M and atomic O in the melt by reaction:
MxOy(s)→xM (in Ti alloy melt)+yO(in Ti alloy melt)
Thus, for the investigation of the thermodynamics of interactions between oxide ceramics and Ti alloy melts, one should not only consider the stability of the oxide itself but also take into account of the solubility of the oxide in Ti alloy melts. The values of the Gibbs free energy of MxOy dissolution (ΔGf) can be obtained by summating the following reactions:
xM(s)+y/2O2→MxOy(s), ΔG1
xM(s)→x[M] in Ti alloy melt, ΔG2
y/2O2→y[O] in Ti alloy melt, ΔG3
namely,
ΔGf=ΔG2+ΔG3-ΔG1 (9)
where ΔG1 is the standard Gibbs free energy of formation of corresponding oxides, as listed in Table 4 [18]; ΔG2 and ΔG3 can be approximately obtained by thermodynamic calculation.
Table 4 Standard Gibbs free energy of formation of chosen oxides [18]

At a given temperature and composition, the Gibbs free energy of M dissolution reaction is expressed as [18]:
![]() (10)
(10)
where i is the component Ti or Al; ai is the activity of i, ai=xiγi; xi is the mole fraction of i,; γi is the activity coefficient of i, as shown in Fig. 2; Mi is the relative atomic mass of solvent i; MM is the relative atomic mass of solute M; ![]() is the coefficient of transformation between different activity standard states, whose value is approximate to unity for an ideal or almost ideal solution and 10-3 for a non-ideal solution, respectively. At a given temperature and composition, the Gibbs free energy of O dissolution reaction is expressed as [18]:
is the coefficient of transformation between different activity standard states, whose value is approximate to unity for an ideal or almost ideal solution and 10-3 for a non-ideal solution, respectively. At a given temperature and composition, the Gibbs free energy of O dissolution reaction is expressed as [18]:
![]() (11)
(11)
where ΔGi is the Gibbs free energy of the oxygen dissolved in the pure i melt, whose value can be approximately obtained by thermodynamic data of binary Ti-O and Al-O systems [19-21], ΔGTi≈-21.5T, ΔGAl≈23.4T. Using Eqs. (9)-(11) and taking one mole oxygen atom as the standard, the Gibbs free energy of MxOy dissolution can be written as:
![]()
![]() (12)
(12)
According to Eq. (12) and thermodynamic data including activity coefficients in Tables 2 and 3, the Gibbs free energy of oxides in Ti alloy melts (0≤x(Al)≤0.5) can be calculated. In this study, four common oxides (CaO, MgO, Al2O3 and Y2O3) are selected for investigation. The calculated results of Gibbs free energy of these oxides in Ti alloy melts (0≤x(Al)≤0.5) are shown in Fig. 3 at different temperatures.
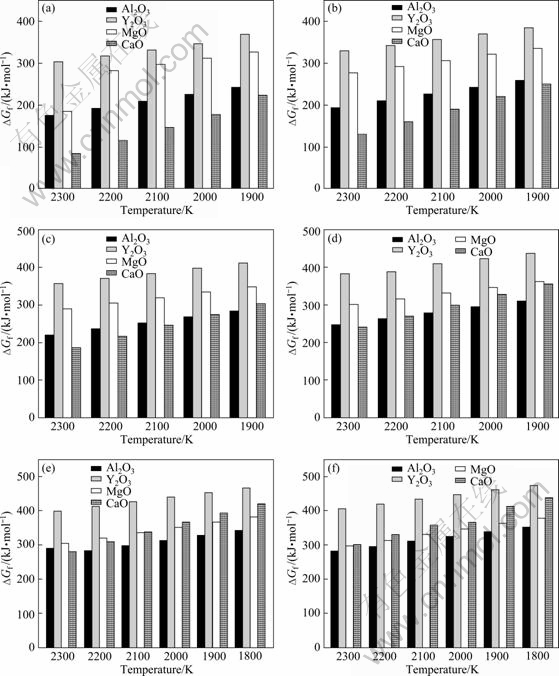
Fig. 3 Calculated Gibbs free energy of four oxides in molten Ti and Ti alloys (0≤x(Al)≤0.5) at different temperatures: (a) In Ti melts; (b) In Ti-10Al melts; (c) In Ti-20Al melts; (d) In Ti-30Al melt; (e) In Ti-40Al melt; (f) In Ti-50Al melt
At equilibrium, the Gibbs free energy of MxOy dissolution is expressed as:

 (13)
(13)
where T is the melt temperature; R is the mole gas constant; a is the chemical activity of components; K is the equilibrium constant; w(M) and w(O) are the mass fractions of M and O content in alloy melts. It can be seen from Eq. (13) that the impurity contents w(M)·w(O) are inversely proportional to the Gibbs free energy of MxOy dissolution (ΔGf ), namely, ln w(M)w(O)∝-ΔGf. This indicates that the higher Gibbs free energy of oxides dissolution leads to the lower equilibrium dissolved impurity contents in alloy melts, consequently indicating the higher thermodynamic stability of oxides. As shown in Fig. 3, both alloy composition and melt temperature are found to directly affect the Gibbs free energy for dissolution of oxides, thus affecting the stability of oxides.
For every figure in Fig. 3, it can be seen that the Gibbs free energy of oxide dissolution is decreased with increasing the melt temperature, indicating that the stability of the oxide decreases with increasing the temperature. This is mainly due to the decrease of Ti and Al activities in melts with decreasing the melt temperature (in Fig. 2), and the chemical reactivity of the melts depends on the activities of Ti and Al elements in melts. Therefore, by decreasing the melt temperature, the chemical reactivity of the melt is directly decreased, and consequently increases the stability of the oxide.
By the comparison of figures in Fig. 3, it can be seen that at the same melt temperature, the Gibbs free energy for dissolution of four oxides increases with increasing the Al content, indicating that the stability of oxides increases with increasing the Al content in the alloy. This is mainly due to the decrease of the activity of Ti with increasing the Al content (in Fig. 2(a)), and the activity changes of Ti directly affect the chemical reactivity of the melts, considering that the chemical reactivity of Ti is higher than that of Al. Therefore, with increasing the Al content of the alloy, the chemical activity of the alloy melt is directly decreased, and consequently increases the stability of the oxide.
In the last two decades, TiAl alloys have attracted attention as potential candidates for high-temperature structural applications in the aerospace, military automotive industry. At the present stage, the main factors to limit the manufacture of mass market TiAl components are the low ductility. Researches so far indicate that the interstitial O content directly affects the room temperature ductility of TiAl alloys. Generally, it is accepted that the oxygen content in cast TiAl alloys should not exceed technically acceptable limit of about 1×10-3. This suggests that one should select oxides with high values of ΔGf as crucible or mould for melting or casting Ti alloys to reduce the alloy contamination. According to the calculated composition—temperature— stability relationship for selected oxides, one can very easily differentiate the relative stability of oxides against Ti alloys with different compositions. For the cast TiAl alloys, the melt temperature is generally lower than 2000 K. Therefore, the order of decreasing stability follows the sequence: when x(Al)≤0.2, Y2O3>MgO>Al2O3>CaO; when 0.2
3.3 Experimental analysis of interactions between Y2O3 ceramics and Ti alloy melts
According to above thermodynamic analysis, one can know that Y2O3 has the highest thermodynamic stability. Therefore, as a representative of evaluated oxides, Y2O3 is selected as crucible materials to run melting experiments of Ti-Al alloys in this work, and the melting result is quantitatively analyzed, as described in Section 2.1.
Figure 4 shows the results of chemical composition analysis of Ti alloy ingots with different Al contents. As shown in Fig. 4, taking into account of the initial oxygen concentration of the master alloy, the oxygen contents of the ingots exposed to the Y2O3 crucibles are higher than that value, and the oxygen content decreases with increasing the Al content in Ti alloys, in agreement with thermodynamic calculations. By comparison of figures in Fig. 3, the Gibbs free energies of dissolution of four oxides increase with increasing the Al content, indicating that the stability of oxides increases with increasing the Al content in the alloy. This is mainly due to the decrease of the Ti activity with increasing the Al content ( Fig. 2(a)), resulting in the weakening of its interaction with the oxide and consequently the decrease of the alloy contamination. Therefore, it is expected that the addition of alloying elements (such as Nb and Cr), which not only improves the comprehensive properties of the alloy but also reduces the Ti activity and decreases the chemical reactivity of the alloy, can help to reduce the oxide-alloy interactions and the alloy contamination. Under the processing conditions used in this work, the oxygen enrichment in Ti alloy melts proportionally increases with increasing the Al content. Regression analysis of experimental data in Fig. 4, yields an equation in the form:
w(O)=-0.233x(Al)+0.1695 (14)
The regression coefficient of this fit is R2=0.99, indicating a good linear relationship between w(O) and x(Al).
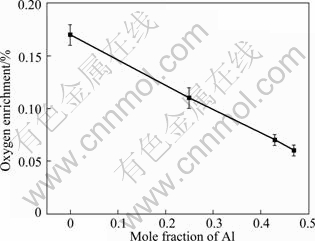
Fig. 4 Results of chemical analysis showing oxygen enrichment of molten Ti alloys with different Al contents at 1973 K
Figure 5 shows the results of chemical composition analysis of Ti-47Al alloy ingots at different melt temperatures. As shown in Fig. 5, taking into account of the initial oxygen concentration of the master alloy, the oxygen contents of the ingots exposed to the Y2O3 crucibles are higher than that value, and the oxygen enrichment increases with increasing the melt temperature, in agreement with the thermodynamic calculations. As shown in Fig. 3(f), the Gibbs free energy values of dissolution of four oxides decrease with increasing the melt temperature, indicating that the stability of oxides decreases with increasing the melt temperature. This is mainly due to the increase of Ti and Al activities in melts with increasing the melt temperature (Fig. 2), resulting in the enhancement of its interaction with the oxide and consequently the increase of the alloy contamination. These findings indicate that the decrease of the melt temperature helps to reduce the oxide-alloy interactions and the alloy contamination. For the processing conditions used in this work, the oxygen enrichment in Ti alloy melts proportionally increases with increasing the melt temperature. Regression analysis of the experimental data in Fig. 5 yields an equation in the form:
w(O)Ti-47Al=0.0002T-0.3346 (15)
The regression coefficient of this fit is R2=0.99, indicating a good linear relationship between w(O) and T.

Fig. 5 Result of chemical analysis showing oxygen enrichment of molten Ti alloys with different melt temperatures
4 Conclusions
1) According to the predicting results of thermodynamic stability of oxides CaO, MgO, Al2O3 and Y2O3 in molten Ti and Ti alloys, it was found that the stability of oxides increased with increasing the Al content in the alloy and decreasing the melt temperature. According to the calculated composition—temperature— stability relationship for evaluated oxides, the order of decreasing stability follows the sequence: when x(Al)≤0.2, Y2O3>MgO>Al2O3>CaO; when 0.2
2) According to the thermodynamic analysis, Y2O3 was found to have the highest thermodynamic stability among the evaluated oxides. As a representative of the evaluated oxides, Y2O3 was selected as crucible materials to run melting experiments of Ti-Al alloys. The melting result was quantitatively analyzed. The results showed that at the same experimental conditions, the oxygen enrichment in Ti alloys proportionally decreased with increasing the Al content in Ti alloys, indicating that the oxide-alloy interaction was reduced with increasing the Al content. For the same alloy composition, the decrease of the melt temperature helps to reduce the oxide-alloy interactions and the alloy contamination.
References
[1] BOYER R R. An overview on the use of titanium in aerospace industry [J]. Materials Science and Engineering A, 1996, 213: 103-114.
[2] SAHA R L, NANDY T K, MISRA R D K, JACOB K T. On the evaluation of stability of rare earth oxides as face coats for investment casting of titanium [J]. Metallurgical and Materials Transactions B, 1990, 21: 559-566.
[3] KIM Y W. Gamma titanium aluminides: Their status and future [J]. JOM, 1995, 7: 39-41.
[4] DING Hong-sheng, GUO Jing-jie, JIA Jun, WANG Chun-yi. Thermodynamic stability of molding materials for casting Ti-6Al-4V alloy [J]. Journal of Harbin Institute of Technology, 1999, 6(2): 56-59.
[5] LIU Ai-hui. The interfacial reaction law and micromechanism between titanium alloy melts and ceramic mould [D]. Harbin: Harbin Institute of Technology, 2007. (in Chinese)
[6] CHEN Yu-yong, XIAO Shu-long, KONG Fan-tao, WANG Xue. Microstructure and interface reaction of investment casting TiAl alloys [J]. Transactions of Nonferrous Metals Society of China, 2006, 16: 1910-1914.
[7] LUO Wen-zhong, SHEN Jun, MIN Zhi-xian, FU Heng-zhi. Investigation of interfacial reactions between TiAl alloy and crucible materials during directional solidification process [J]. Rare Metal Materials and Engineering, 2009, 38: 1441-1445. (in Chinese)
[8] LI Bang-sheng, LIU Ai-hui, NAN Hai, BI Wei-sheng, GUO Jing-jie, FU Heng-zhi. Wettability of TiAl alloy melt on ceramic moulds in electromagnetic field [J]. Transactions of Nonferrous Metals Society of China, 2008, 18: 518-522.
[9] JIA Qing, CUI Yu-you, YANG Rui. A study of two refractories as mould materials for investment casting TiAl based alloys [J]. Journal of Materials Science, 2006, 41: 3045-3049.
[10] LIU Ai-hui, LI Bang-sheng, NAN Hai, SUI Yan-wei, GUO Jing-jie, FU Heng-zhi. Study of interfacial reaction between TiAl alloys and four ceramic molds [J]. Rare Metal Materials and Engineering, 2008, 37: 956-959. (in Chinese)
[11] ZHU Yan, YANG Yan-qing, SUN Jun. Calculation of activity coefficients for components in ternary Ti alloys and intermetallics as matrix of composites [J]. Transactions of Nonferrous Metals Society of China, 2004, 14: 875-879.
[12] GUO Jing-jie, SU Yan-qing. The themodynamic and kinetic analysis of Ti alloys during the ISM process [M]. Harbin: Harbin Institute of Technology Press, 1998. (in Chinese)
[13] DING Xue-yong, FAN Peng, HAN Qi-yong. Models of activity and activity interaction parameter in ternary metallic melt [J]. Acta Metallurgica Sinica, 1994, 30(2): 49-59. (in Chinese)
[14] DING Xue-yong, WANG Wen-zhong. A new thermodynamic calculation formula for activity of component in binary system [J]. Acta Metallurgica Sinica, 1994, 30(10): 444-447. (in Chinese)
[15] MIDEMA A R, DE CHATEL P F, DE BOER F R. Cohesion in alloys fundamentals of a semi-empirical model [J]. Physica B, 1980, 100: 1-28.
[16] BARBOSA J, RIBEIRO C S, MONTEIRO A C. Influence of superheating on casting of γ-TiAl [J]. Intermetallics, 2007, 15: 945-955.
[17] KUANG J P, HARDING R A, CAMPBELL J. Investigation into refractories as crucible and mould materials for melting and casting γ-TiAl alloys [J]. Materials Science and Technology, 2000, 16: 1007-1016.
[18] HUANG Xi-gu. Metallurgical principles [M]. Beijing: Metallurgical Industry Press, 1989. (in Chinese)
[19] WALDNER P. Modelling of oxygen solubility in titanium [J]. Scripta Materialia, 1999, 40: 969-974.
[20] CANCAREVIC M, ZINKEVICH M, ALDINGER F. Thermodynamic modeling of the system titanium-oxygen [J]. Calphad, 2007, 31: 330-342.
[21] DAS K, CHOUDHURY P, DAS S. The Al-O-Ti (Aluminum- oxygen-titanium) system [J]. Journal of Phase Equilibria, 2002, 23: 525-536.
崔仁杰,唐晓霞,高 明,张 虎,宫声凯
北京航空航天大学 材料科学与工程学院 空天材料与服役教育部重点实验室,北京 100191
摘 要:研究CaO、MgO、Al2O3和Y2O3与熔融Ti及Ti合金之间的界面反应热力学。根据氧化物在Ti合金熔体中的溶解反应机制建立热力学模型,通过热力学分析预测各氧化物相对于Ti-Al(0≤x(Al)≤0.5)合金的热力学稳定性。根据氧化物坩埚熔炼实验结果来研究氧化物与Ti-xAl合金熔体的界面反应,对熔炼效果进行定量分析,发现随着合金中Al含量的提高和熔体温度的降低,合金中杂质元素含量成比例的减少,这与氧化物稳定性计算结果一致。
关键词:Ti合金;热力学;氧化物陶瓷;界面反应
(Edited by YANG Hua)
Corresponding author: ZHANG Hu; Tel: +86-10-82316958; E-mail: zhanghu@buaa.edu.cn
DOI: 10.1016/S1003-6326(11)61261-2

