
J. Cent. South Univ. (2019) 26: 515-523
DOI: https://doi.org/10.1007/s11771-019-4023-9

Molecularly imprinted polymer based on upconversion nanoparticles for highly selective and sensitive determination of Ochratoxin A
YAN Zhen(闫祯), FANG Guo-zhen(方国臻)
State Key Laboratory of Food Nutrition and Safety, Tianjin University of Science and Technology,Tianjin 300457, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract: A novel molecularly imprinted polymer (MIP) based on upconversion nanoparticles (UCNPs) was successfully synthesized for determination of Ochratoxin A (OTA). The MIP was developed on the silica-coated UCNPs using N-(1-hydroxy-2-naphthoyl amido)-(L)-phenylalanine (HNA-Phe) as the alternative template. The final composite combined the advantages of the high selectivity of MIP with the high fluorescence intensity of UCNPs which was selective and sensitive to OTA. Under the optimal condition, the fluorescence intensity of UCNPs@SiO2@MIP decreases linearly when the concentration of OTA increases from 0.05 to 1.0 mg/L. The detection limit of OTA with the method was 0.031 mg/L. At three spiked concentration levels (50, 100 and 200 μg/kg), the recovery ranges of OTA in corn, rice and feed are 88.0%–91.6%, 80.2%–91.6% and 89.2%–90.4%, respectively.
Key words: molecularly imprinted polymer; upconversion nanoparticles; fluorescent sensing; Ochratoxin A
Cite this article as: YAN Zhen, FANG Guo-zhen. Molecularly imprinted polymer based on upconversion nanoparticles for highly selective and sensitive determination of Ochratoxin A [J]. Journal of Central South University, 2019, 26(3): 515–523. DOI: https://doi.org/10.1007/s11771-019-4023-9.
1 Introduction
Ochratoxin A (OTA) is a toxic metabolite produced by some strains of aspergillus and penicillium [1]. OTA has a serious health hazard to animals and humans, mainly including nephrotoxic, hepatotoxic, immune-toxic, teratogenic and carcinogenic effects [2, 3]. The authorities define the OTA maximum limit because OTA widely exists in a variety of food, such as feed, beans, wheat and beverages [4, 5]. Several analytical methods [6, 7] for detecting the presence of OTA residues in biological fluids and food have been reported, including thin-layer chromatography (TLC), liquid chromatography (LC), gas chromatography (GC), enzyme-linked immunosorbent assay (ELISA) and capillary electrophoresis (CE). Among these methods, ELISA method is frequently used, but it is well known biological antibodies are costly and low stable in the ELISA preparation process. The HPLC with fluorescence detector (FLD) is commonly used as low detection limits can be reached. Because of the complexity of food matrix and the low concentration of OTA, the analytical method generally requires the complicated extraction and purification of OTA such as solid-phase extraction (SPE) before determination by high performance liquid chromatography (HPLC). Accordingly, there is a considerable interest in developing an effective and specific material for extracting target analytes from complex food matrices. And fortunately, molecularly imprinted polymer (MIP) provides a good choice with outstanding advantages in good selectivity, low cost, easy synthesis and reuse [8]. Compared to traditional SPE adsorbents, MIP has drawn more and more attentions for the complex samples analysis because of the advanced specificity [9]. To date, several examples of MIP binding the OTA have been reported [10, 11].
Molecular imprinting is used to prepare the polymers with special recognition sites for the templates or their analogs [12, 13]. MIPs prepared with traditional method are exposed to some problems, including relative positions of the binding sites, the elution of the template and selective absorbance. However, surface molecular imprinting polymer has potential to overcome shortcomings, and increases surface area as well as rebinding capacity [14]. In recent years, many researchers have proposed a new polymer that was prepared based on fluorescent materials. FANG [15] reported a feasible method for preparing MIP on quantum dots (QDs) for selectivity recognition of the Zearalenone (ZON). XU et al [16] introduced green carbon dots into polymerization system to develop a fluorescent probe, which exhibits high sensitivity and selectivity toward sterigmatocystin. However, most of the developed MIP-based probes were excited in UV/visible light, inducing the auto- fluorescence from biological samples and limited light penetration depth [17]. Further, the sensitivity of fluorescence detection will be impaired to some extent.
With the development of highly fluorescent materials, UCNPs have drawn many researchers attention [18–21]. UCNPs are able to convert near infrared (NIR) light to UV-visible light via energy transfers or absorptions of multiple photon [22]. Due to the above advantages, UCNPs are widely used in fields of optical amplifier, bioimaging and bio-probe [23]. MIP-coated UCNPs have been successfully applied as a fluorescent sensor to detect molecular and biomolecular templates. QIAN et al [24] developed a novel sensitive core-shell UCNPs@MIP for detecting metolcarb. In the sensing system, the MIP provided special binding sites and selective recognition for the target analytes, and the UCNPs acted as fluorophores to provide fluorescent signals. The method can achieve qualitative and quantitative analysis to the target by measuring the fluorescence change. Therefore, it is very significant for the sensitive fluorescent sensor in future applications.
In this study, a novel method using MIP-coated UCNPs for the specific recognition of OTA was proposed. The fluorescent sensor was prepared with HNA-Phe [25] as the alternative template, 3-aminopropyltriethoxylsilane (APTES) as functional monomer, UCNPs@SiO2 as support vector and tetraethoxysilane (TEOS) as cross-linker in the imprinting process. The response of fluorescence intensity and the characterization of the UCNPs@SiO2@MIP were described in detail below.
2 Experimental
2.1 Materials
All reagents in the whole experiment were the analytical grade at least. Y(NO3)3·6H2O, Yb(NO3)3·5H2O, Er(NO3)3·5H2O, Ochratoxin A (OTA), Ochratoxin B (OTB) and Aflatoxin B1(AFB1) were purchased from Sigma Aldrich St Louis USA. Triton X-100 and Oleic acid (technical grade, 90%) were purchased from Sinopharm Chemical Reagent Co. Ltd. 3-aminopropyltriethoxysilane (APTES) and Tetraethoxysilane (TEOS) were purchased from Alfa Aesar Co. Ltd. (Tianjin, China). Corn, feed and rice were purchased from a local supermarket (Tianjin, China).
2.2 Characterizations
Fluorescence measurements were carried out with an F-4500 fluorescence spectrofluorometer (HITACHI, Japan) equipped with an external 980 nm diode laser. Fourier transform infrared (FT-IR) spectra were recorded by a Tensor 22 FT-IR spectrophotometer (Bruker, Germany). The size, shape and surface morphology was observed by scanning electron microscopy (SEM; SU1510; Hitachi, Japan). Transmission electron microscopy (TEM) was carried out with JEOL, Tokyo, Japan. X-ray powder diffraction (XRD) patterns were performed with a D8 X-ray diffractometer (Bruker- AXS, Germany). HPLC was recorded on LC-20AB, Shimadzu, Japan.
2.3 Synthesis of UCNPS@SiO2
2.3.1 Synthesis of UCNPs
The NaYF4 doped with Yb3+, Er3+ used in the paper was prepared according to the reported literature [26]. In a 100 mL flask, 0.7 g NaOH,5.0 mL H2O were well mixed 8.0 mL ethanol and with 8.0 mL oleic acid to form a yellow translucent solution at room temperature. 2.5 mL 0.4 mol/L Re(NO3)3 (2 wt% Er3+ and 20 wt% Yb3+) solution was dropped into the yellow translucent solution with continually stirring till a white viscous solution was formed. 20 mL mixture solution containing NH4F (247 mg), H2O (10 mL) and ethanol (10 mL) was added with vigorous stirring for 10 min. After sonicated for 30 min, the final mixture was put into a 100 mL Teflon–lined autoclave, then annealed at 230 °C for 12 h. After cooling down naturally, the resulted UCNPs were purified with ethanol for three times. And then activated with Trion X-100/H2O (1:4, v/v), filtered and dried, to obtain the activated UCNPs for subsequent experiments.
2.3.2 Modification of UCNPs
The activated UCNPs were modified by silica according to the reported procedure [27]. 100 mg of the above activated UCNPs was dissolved in 150 mL ethanol and sonicated for 30 min. 40 mL H2O and 2 mL ammonia (28 wt%) were added with stirring at 30 °C for 10 min, to get a transparent emulsion. The TEOS (60 mg) mixed with ethanol (10 mL) was added dropwise with stirring for 6 h. After washing three times with ethanol and water, respectively, the UCNPS@SiO2 nanocrystals were obtained.
2.4 Synthesis of UCNPs@SiO2@MIP
1 mmol of mimic template HNA-Phe was dissolved in a mixture of methanol (2.5 mL). APTES (functional monomer, 0.936 mL) was added and continually stirred for 20 min. 100 mg UCNPS@SiO2 was added with stirring for 50 min. Subsequently, TEOS (cross-linker, 0.134 mL) and 0.1 mol/L CH3COOH (1 mL) were added, and stirred at 30 ○C for 12 h in dark condition. After polymerization, the polymer was washed with methanol–acetic acid (9:1, v/v) and washed by methanol until the template was not detected by UV–vis spectrophotometry. Finally, the obtained UCNPs@SiO2@MIP was successfully prepared after drying under vacuum at 50 °C for 12 h. The non-imprinted polymer (UCNPs@SiO2@NIP) was prepared with the same process in the absence of HNA-Phe.
2.5 Characterization of UCNPS@SiO2@MIP
In the study, all the fluorescence measurements were carried out with an external laser of 980 nm wavelength. The external diode laser was 1 W. To study the adsorption capacity of UCNPS@SiO2@MIP, 1 mg UCNPS@SiO2@MIP and UCNPS@SiO2@NIP were dispersed in 3 mL 1 mg/L OTA-acetone with different time in 4 mL quartz cell, respectively. After fully shook, the fluorescence intensity of every solution was measured. The specificity experiments were performed using AFB1 and OTB as competitors. 1 mg UCNPS@SiO2@MIP or UCNPS@SiO2@NIP was well dispersed in 3 mL acetone solution of OTA, AFB1 or OTB, and shook at room temperature for 3 h, then determined quickly.
2.6 Preparation of samples
The UCNPs@SiO2@MIP fluorescent method was used to detect OTA in corn, rice and feed. The standard addition recovery experiments were performed by adding OTA at three different levels 50, 100 and 200 μg/kg to the real samples, the mixture was placed for 12 h before preprocessing. The extraction step of OTA in the samples was shown as follows: 25 mL of the solution (acetonitrile/H2O, 6:4, v/v), 0.5 g NaCl and 5 g samples were vigorously shook for 5 min, then ultrasonically extracted for 20 min, stood and filtered. The collection was evaporated till dryness. The remaining residue was dissolved with acetone, then filtered with a 0.22 μm filter for subsequent analysis.
2.7 HPLC analysis
Traditional HPLC was used to verify the results of the proposed method for OTA detection. Prior to HPLC analysis, the collection purification experiment was carried out by SPE with a commercial OTA immunoaffinity column (3 mL, Pribo Fast). In the HPLC system, excitation wavelength (Ex) and emission wavelength (Em) were set at 333 nm and 460 nm, respectively. The separations were dependent on a C18 column (4.6 mm×150 mm, 5 μm, Waters Symmetry, USA). The mobile phase with acetonitrile/water/acetic acid (48:51:1, v/v/v) was at a flow rate of 1.0 mL/min at room temperature.
3 Results and discussion
3.1 Preparation of UCNPs@SiO2@MIP
The study proposed a novel method SiO2 modified UCNPs to detect OTA. The synthesis process and the mechanism of OTA detection were illustrated in Figure 1. In the polymerization process, MIP was prepared based on UCNPs@SiO2 with APTES and TEOS as the functional monomer and cross-linker, respectively. The polymerization was successfully prepared by the formed hydrogen bonds between HNA-Phe and the APTES. After the template molecules were removed, UCNPs@SiO2@MIP with specific recognition sites was formed. The fluorescence intensity of UCNPs@SiO2@MIP was recovered as the template was extracted. The template is present in the UCNPs@SiO2@MIP, the fluorescence of UCNPs@SiO2@MIP is quenched.
3.2 Characterization of sensing material
3.2.1 SEM and TEM characterization of sensing material
The SEM image of UCNPs@SiO2 is shown in Figure 2(a). The UCNPs@SiO2 is uniformly sized with 200 nm in the diameters and lengths of about 2 μm. The TEM image in Figure 2(b) shows that the thickness of SiO2 layer is 7 nm.
The SEM images of UCNPs@SiO2@MIP and UCNPs@SiO2@NIP are shown in Figures 2(c) and 2(d), respectively. Figure 2(c) shows that UCNPs@SiO2@MIP particles have the uniform diameters. The comparison clearly shows that UCNPs@SiO2@NIP diameter is not as uniform as UCNPs@SiO2@MIP. The size of UCNPs@SiO2@ MIP is larger than that of UCNPs@SiO2@NIP, because the template is added in the imprinting process.

Figure 1 Synthesis process for UCNPs@SiO2@MIP
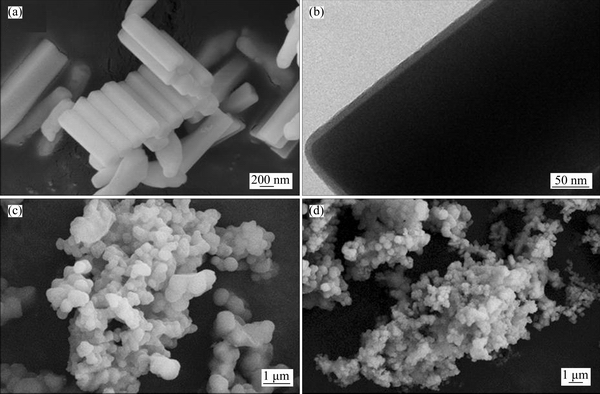
Figure 2 SEM image (a) and TEM image (b) of UCNPs@SiO2, SEM images of UCNPs@SiO2@MIP (c) and UCNPs@SiO2@NIP (d)
3.2.2 XRD characterization of sensing material
The XRD patterns of UCNPs and UCNPs@SiO2@MIP are shown in Figure 3. The structure UNCPs is in accord with the JCPDS card 16-0334, which is hexagonal phase. It is obvious that UCNPs@SiO2@MIP and the UCNPs have the similar XRD pattern, illustrating the structures of UCNPs@SiO2@MIP and UCNPs are the same. However, the pattern of UCNPs@SiO2@MIP is lower responded compared with that of UCNPs, showing UCNPs@SiO2@MIP is successfully prepared.

Figure 3 XRD patterns of UCNPs and UCNPs@SiO2@ MIP:
3.2.3 FT-IR analysis of sensing material
FT-IR spectroscopies of the UCNPs, UCNPs@SiO2@MIP and UCNPs@SiO2@NIP are shown and compared in Figure 4. From Figures 4(b) and (c), it can be seen that peaks at 1098 cm-1 and 958 cm-1 are caused by the stretching vibration of Si—O—Si and Si—OH, respectively. The bending vibrations of Si—O are at 465 cm–1 and 799 cm–1. The stretching vibrations of C—H and CH2—N can be found at near 1556 cm–1 and 1443 cm–1. These results confirm that the polymer has been synthesized successfully. Compared to UCNPs@SiO2@MIP (Figure 4(b)), UCNPs@ SiO2@NIP (Figure 4(c)) shows some similar peaks, which demonstrates that the HNA-Phe has been removed thoroughly after the elution.
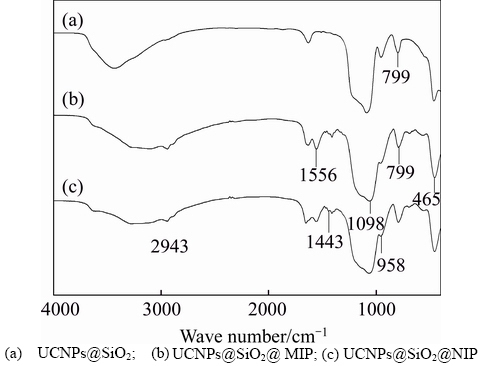
Figure 4 FT-IR spectra:
3.3 Response of UCNPs@SiO2@MIP to OTA
To evaluate the fluorescence response of the UCNPs@SiO2@MIP to OTA, adsorption experiments were performed at different OTA concentrations. Figure 5 shows the fluorescence intensity of UCNPs@SiO2@MIP and UCNPs@ SiO2@NIP with the concentration of OTA from 0 to 1 mg/L. Figure 5(a) shows the fluorescence intensity of the UCNPs@SiO2@MIP decreases as the OTA concentration increases. In addition, at the same OTA concentration, the change of fluorescence intensity of the UCNPs@ SiO2@MIP is more significant than that of UCNPs@SiO2@NIP, as shown in Figure 5.
The fluorescence intensity decreases mainly because the hydrogen bonding is formed between UCNPs@SiO2@MIP and OTA in rebinding process. The fluorescence quenching of UCNPs@SiO2@ MIP and UCNPs@SiO2@NIP conforms to the Stern–Volmer equation [28]
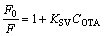 (1)
(1)
where F is the fluorescence intensity of UCNPs@SiO2@MIP in the presence of OTA; F0 refers to the fluorescence intensity of UCNPs@SiO2@MIP in the absence of OTA; KSV denotes the Stern–Volmer constant, and COTA represents the concentration of OTA. The imprinting factor (IF, KSV,MIP/KSV,NIP) is used to evaluate the selectivity of the UCNPs@SiO2@MIP. Under the optimum condition, the IF is 2.32, showing that the UCNPs@SiO2@MIP can effectively recognize OTA.
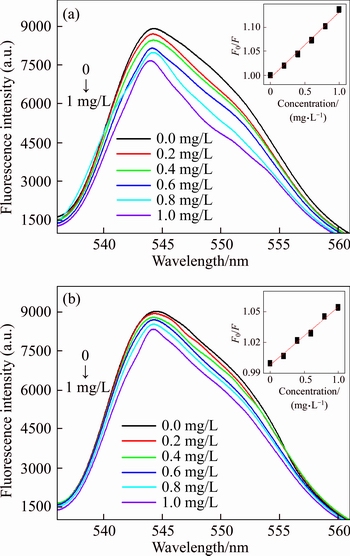
Figure 5 Fluorescence intensity of UCNPs@SiO2@MIP (a) and UCNPs@SiO2@NIP (b) at different concentrations of OTA
To investigate optimal adsorption time, 1 mg/L of OTA is chosen in the kinetics experiment of UCNPs@SiO2@MIP. Figure 6 shows the fluorescence intensity is monotonous increasing in the first 2 h. When the adsorption time is 2.5 h, there is no significant change in the fluorescence intensity of UCNPs@SiO2@MIP. Furthermore, the F0/F of the UCNPs@SiO2@MIP is higher than that of UCNPs@SiO2@NIP, because the cavities matched OTA are formed on the surface of the UCNPs@SiO2@MIP. Therefore, the adsorption equilibrium time is 2.5 h.
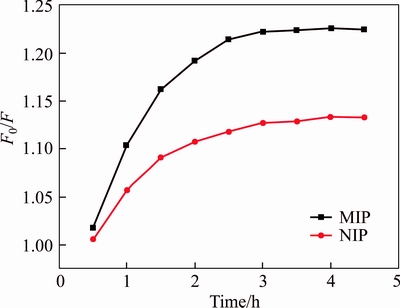
Figure 6 Adsorption kinetics of UCNPs@SiO2@MIP and UCNPs@SiO2@NIP
3.4 Specificity study
To investigate the selectivity of the UCNPs@SiO2@MIP to OTA, competitive binding was carried on by using the AFB1 and OTB as competitive toxin, as shown in Figure 7(a).Figure 7(a) shows the fluorescence of UCNPs@ SiO2@MIP and UCNPs@SiO2@NIP quench in the presence of AFB1, OTA and OTB, respectively. UCNPs@SiO2@MIP shows higher selectivity for the mixture (OTA, OTB and AFB1) than other interferents in Figure 7(b). Compared to OTA and OTB, AFB1 slightly quenches the fluorescence responses of UCNPs@SiO2@MIP, because of HNA-Phe as the template in imprinting process, AFB1, HNA-Phe and OTA have a great difference in shape, size and functional groups, which results in the poor selectivity to AFB1. The selectivity of UCNPs@SiO2@MIP for OTA and OTB is almost no different, due to the similar structures. However, little OTB usually exists in contaminated samples. If there is interference, the question of OTB can be solved after the extraction process. It is noteworthy that OTA, OTB and AFB1 have caused a slight change of the fluorescence of the UCNPs@SiO2@NIP, respectively, possibly because of physical adsorption. In all, UCNPs@SiO2@MIP is a selective and sensitive recognition to OTA.

Figure 7 Selective adsorption (a) and competitive adsorption (b) of OTA, AFB1 and OTB by UCNPs@SiO2@MIP and UCNPs@SiO2@NIP
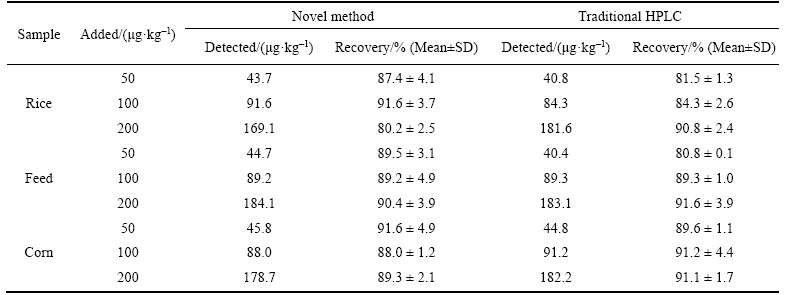
Table 1 Recoveries results of samples spiked with OTA determined by novel method and traditional HPLC (n=3)
3.5 Detection range and limit
The detection range and detection limit are studied to demonstrate the analytical performance of UCNPs@SiO2@MIP. Figure 5(a) shows the fluorescence intensity of UCNPs@SiO2@MIP decreases notably with the increasing concentration
of OTA. When OTA concentration increases from 0.05 mg/L to 1.0 mg/L, the relative fluorescence intensity of UCNPs@SiO2@MIP decreases linearly with a correlation coefficient of 0.991. The detection limit is 0.031 mg/L, the precision for nine replicate measurements of 1 mg/L OTA is 2.48% (RSD).
3.6 Sample analysis and HPLC validation
The accuracy and validity of the proposed method for the detection of OTA is determined by the standard addition recovery experiments. The results of the recovery study are listed in Table 1. The UCNPs@SiO2@MIP method shows that recoveries range from 80.2% to 91.6% for three samples, while the recoveries range of 80.8%– 91.6% is determined by the traditional HPLC method. The results of standard addition recovery experiments show that the novel method is feasible for determination of OTA in the real samples.
4 Conclusions
1) A novel fluorescent sensor based on β-NaYF4: Yb3+, Er3+ is successfully prepared for detecting OTA. The fluorescent sensor combines the advantages of UCNPs and MIP, which exhibits high sensitivity and specific recognition to OTA.
2) The fluorescence intensity of UCNPs@SiO2@MIP decreases linearly as the concentration of OTA increases from 0.05 mg/L to 1.0 mg/L.
3) UCNPs@SiO2@MIP is applied to determining OTA in corn, rice and feed. At the spiked concentration levels of 50, 100 and 200 μg/kg, the recoveries range from 80.2% to 91.6% for three samples. Therefore, the proposed method is very promising for the detection of OTA.
References
[1] OZCAN Z, GUL G, YAMAN I. Ochratoxin A activates opposing c-MET/PI3K/Akt and MAPK/ERK 1-2 pathways in human proximal tubule HK-2 cells [J]. Archives of Toxicology, 2015, 89(8): 1313–1327. DOI: 10.1007/s00204- 014-1311-x.
[2] MANTLE P G. Rat kidney cancers determined by dietary Ochratoxin A in the first year of life [J]. Journal of Kidney Cancer & VHL, 2016, 3(3): 1–10. DOI: 10.15586/ jkcvhl. 2016. 58.
[3] XIONG Ke, WANG Xiao-ling, ZHI Hui-wei, SUN Bao-guo, LI Xiu-ting. Identification and safety evaluation of a product from biodegradation of ochratoxin a by an aspergillus strain [J]. Journal of the Science of Food & Agriculture, 2016, 97(2): 434–443. DOI: 10.1002/jsfa.7742.
[4] WOO C S J, EL-NEZAMI H. Maternal-fetal cancer risk assessment of Ochratoxin A during pregnancy [J]. Toxins, 2016, 8(4): 1–16. DOI: 10.3390/ toxins8040087.
[5] PALUMBO J D, O'KEEFFE T L, HO Y S, SANTILLAN C J. Occurrence of Ochratoxin A contamination and detection of Ochratoxigenic aspergillus species in retail samples of dried fruits and nuts [J]. Journal of Food Protection, 2015, 78(4): 836–842. DOI: 10.4315/0362-028X.JFP-14-471.
[6] OKUMA T A, HUYNH T P, HELLBERG R S. Use of enzyme-linked immunosorbent assay to screen for aflatoxins, Ochratoxin A, and deoxynivalenol in dry pet foods [J]. Mycotoxin Research, 2018, 34(1): 69–75. DOI: https://doi.org/ 10.1007/ s12550-017-0300-3.
[7] LAU B P Y, SCOTT P M, LEWIS D A, KANHERE S R. Quantitative determination of Ochratoxin A by liquid chromatography/electrospray tandem mass spectrometry [J]. Journal of Mass Spectrometry, 2000, 35(1): 23–32. DOI: 10.1002/(SICI)1096-9888(200001)35:1<23::AID-JMS903>3.0.CO;2-B.
[8] WANG Pei-long, SUN Xiao-hua, SU Xiao-ou, WANG Tie. Advancements of molecularly imprinted polymers in the food safety field [J]. Analyst, 2016, 141(12): 3540–3553. DOI: 10.1039/c5an01993a.
[9] NEZHADALI A, FEIZY J, BEHESHTI H R. A molecularly imprinted polymer for the selective extraction and determination of fenvalerate from food samples using high-performance liquid chromatography [J]. Food Analytical Methods, 2015, 8(5): 1225–1237. DOI: 10.1007/ s12161-014-0004-7.
[10] YOLA M L, GUPTA V K, ATAR N. New molecular imprinted voltammetric sensor for determination of Ochratoxin A [J]. Materials Science & Engineering C, 2016, 61: 368–375. DOI: 10.1016/j.msec.2015.12.057.
[11] CAO Ji-liang, KONG Wei-jun, ZHOU Shu-jun, YIN Li-hui, WAN Li, YANG Mei-hua. Molecularly imprinted polymer-based solid phase clean-up for analysis of Ochratoxin A in beer, red wine, and grape juice [J]. Journal of Separation Science, 2013, 36(7): 1291–1297. DOI: 10.1002/ jssc.201201055.
[12] HONG Chao-ma, LI Hai-pu, YANG Zhao-guang, XU Qian-jin, MA Li. Preparation and property of environmental hormone 17β-estradiol molecularly imprinted polymers [J]. Journal of Central South University: Science and Techaology, 2014, 45(5): 1403–1410. (in Chinese)
[13] ZHAN Jie, FANG Guo-zhen, YAN Zhen, PAN Ming-fei, LIU Cui-cui. Preparation of a semicovalent, molecularly surface imprinted polymer for the rapid determination of trace acid orange II in food and environmental samples [J]. Analytical & Bioanalytical Chemistry, 2013, 405(19): 6353–6363. DOI: 10.1007/s00216-013-7036-5.
[14] YANG Yan, MENG Xiang-jun, XIAO Zheng-gang. Synthesis of a surface molecular imprinting polymer based on silica and its application in the identification of nitrocellulose [J]. Rsc Advances, 2018, 8(18): 9802–9811. DOI: 10.1039/c7ra13264f.
[15] FANG Guo-zhen, FAN Chao, LIU Hui-lin, PAN Ming-fei, ZHU Hui-dan, WANG Shuo. A novel molecularly imprinted polymer on CdSe/ZnS quantum dots for highly selective optosensing of mycotoxin zearalenone in cereal samples [J]. Rsc Advances, 2013, 4(6): 2764–2771. DOI: 10.1039/c2jm33522k.
[16] XU Long-hua, FANG Guo-zhen, PAN Ming-fei, WANG Xue-feng, WANG Shuo. One-pot synthesis of carbon dots-embedded molecularly imprinted polymer for specific recognition of sterigmatocystin in grains [J]. Biosens Bioelectron, 2016, 77: 950–956. DOI: 10.1016/j.bios.2015. 10.072.
[17] GUO Ting, DENG Qi-liang, FANG Guo-zhen, LIU Cui-cui, HUANG Xuan, WANG Shuo. Molecularly imprinted upconversion nanoparticles for highly selective and sensitive sensing of Cytochrome c [J]. Biosensors & Bioelectronics, 2015, 74: 498–503. DOI: 10.1016/j.bios.2015.06.058.
[18] GNACH A, LIPINSKI T, BEDNARKIEWICZ A, RYBKA J, CAPOBIANCO J A. Upconverting nanoparticles: Assessing the toxicity [J]. Chemical Society Reviews, 2015, 44(6): 1561–1584. DOI: 10.1039/c4cs00177j.
[19] LIU Yu-jia, LU Yi-qing, YANG Xu-san, ZHENG Xian-lin, WEN Shi-hui, WANG Fan, VIDAL X, ZHAO Jiang-bo, LIU De-ming, ZHOU Zhi-guang. Amplified stimulated emission in upconversion nanoparticles for super-resolution nanoscopy [J]. Nature, 2017, 543(7644): 229–233. DOI: 10.1038/nature21366.
[20] LV Jia-jia, ZHAO Sen, WU Shi-jia, WANG Zhou-ping. Upconversion nanoparticles grafted molybdenum disulfide nanosheets platform for microcystin-LR sensing [J]. Biosensors & Bioelectronics, 2017, 90(15): 203–209. DOI: 10.1016/j.bios.2016.09.110.
[21] CHEN Guo-jun, RENATA J S, ESQUIBEL C R, LOU I, ZHENG Qi-feng, DAMMALAPATI A, HARRISON A, ELICEIRI K W, TANG Wei-ping, CHEN H. Neuroendocrine Tumor-targeted upconversion nanoparticle-based micelles for simultaneous nir-controlled combination chemotherapy and photodynamic therapy, and fluorescence imaging [J]. Advanced Functional Materials, 2017, 27(8): 1–13. DOI: 10.1002/adfm.201604671.
[22] WU S, BUTT H J. Near-infrared-sensitive materials based on upconverting nanoparticles [J]. Advanced Materials, 2016, 28(6): 1208–1226. DOI: 10.1002/adma.201502843.
[23] LIU Xiao-lin, ZHANG Ning, LI Dan, LI Zhi-cheng, YOU Wei-xiong, ZHANG Qian, XIA Li-bin, YANG Bin. Fuel combustion synthesis and upconversion properties of Yb3+ and Er3+ dual-doped ZrO2 nanocrystals [J]. Journal of Central South University, 2017, 24(10): 2209–2214. DOI: 10.1007/ s11771-017-3629-z.
[24] QIAN Kun, FANG Guo-zhen, WANG Shuo. Highly sensitive and selective novel core–shell molecularly imprinted polymer based on NaYF4: Yb3+, Er3+ upconversion fluorescent nanorods [J]. Rsc Advances, 2013, 3(12): 3825–3828. DOI: 10.1039/c3ra23003a.
[25] BAGGIANI C, BIAGIOLI F, ANFOSSI L, GIOVANNOLI C, PASSINI C, GIRAUDI G. Effect of the mimic structure on the molecular recognition properties of molecularly imprinted polymers for Ochratoxin A prepared by a fragmental approach [J]. Reactive & Functional Polymers, 2013, 73(6): 833–837. DOI: 10.1016/j.reactfunctpolym. 2013.03.018.
[26] ZHANG Fang, WAN Ying, YU Ting, ZHANG Fu-qiang, SHI Yi-feng, XIE Song-hai, LI Yi-gang, XU Lei, TU Bo, ZHAO Dong-yuan. Uniform nanostructured arrays of sodium rare-earth fluorides for highly efficient multicolor upconversion luminescence [J]. Angewandte Chemie International Edition, 2007, 119(42): 7976–7979. DOI: 10.1002/anie.200702519.
[27] MADER H S, LINK M, ACHATZ D E , UHLMANN K , LI Xiao-hua, WOLFBEIS O S. Surface-modified upconverting microparticles and nanoparticles for use in click chemistries [J]. Chemistry-A European Journal, 2010, 16(18): 5416– 5424. DOI: 10.1002/chem.201000117.
[28] LIU Hui-lin, FANG Guo-zhen, ZHU Hui-dan, LI Chang-mo, LIU Cui-cui, WANG Shuo. A novel ionic liquid stabilized molecularly imprinted optosensing material based on quantum dots and graphene oxide for specific recognition of vitamin E [J]. Biosensors & Bioelectronics, 2013, 47(10): 127–132. DOI: https://doi.org/10.1016/j.bios.2013.03.006.
(Edited by FANG Jing-hua)
中文导读
上转换荧光传感检测赭曲霉毒素A的研究
摘要:本文基于分子印迹技术的高选择性和上转换纳米材料的荧光特性,以赭曲霉毒素A(OTA)的结构类似物HNA-Phe为模板,开发了一种能高选择、高灵敏地识别痕量OTA的分子印迹聚合物(UCNPs@SiO2@MIP)。在最优条件下,当OTA浓度为0.05~1.0 mg/L时,荧光印迹聚合物的荧光猝灭程度与OTA的浓度呈现良好的线性关系。建立了OTA的荧光传感检测方法,该方法的最低检出限为0.031 mg/L。当OTA的加标浓度为50、100和200 μg/kg 时,大米、玉米和饲料中OTA的回收范围分别为80.2%~91.6% (RSD<4.6%)、89.2%~90.4% (RSD<5.5%) 和88.0%~91.6% (RSD<5.4%)。
关键词:分子印迹聚合物;上转换纳米材料;荧光传感;赭曲霉毒素A
Foundation item: Project(17ZYPTJC00050) supported by Science and Technology Committee of Tianjin, China; Project(2017YFC1600803) supported by the Ministry of Science and Technology of China
Received date: 2018-01-25; Accepted date: 2018-09-03
Corresponding author: FANG Guo-zhen, PhD, Professor; Tel: +86-22-60912493; E-mail: fangguozhen@tust.edu.cn; ORCID: 0000- 0001-5346-953X