Article ID: 1003-6326(2005)03-0666-05
Densification of Ni-NiFe2O4 cermets for aluminum electrolysis
LAI Yan-qing(赖延清), SUN Xiao-gang(孙小刚),
LI Jie(李 劼), DUAN Hua-nan(段华南),
LI Xin-zheng(李新征), ZHANG Gang(张 刚), TIAN Zhong-liang(田忠良)
(School of Metallurgical Science and Engineering, Central South University,
Changsha 410083, China)
Abstract: The density of cermet inert anodes in aluminum electrolysis is of great importance. Ni-NiFe2O4 cermets were studied with respect to their densification affected by ball milling time, particle size of raw powders, contents of metallic phase, sintering atmosphere and temperature. The results show that, prolonging ball milling time will increase the density with the optimum value of 150min; cermets containing 0-15%Ni(mass fraction) have high relative density ranging from 94% to 96%, but with Ni content increasing, the density slightly decreases; weak reductive atmosphere is favorable to densification; the relative density increases from 80.38% to 96.85% with the sintering temperature increasing from 1100℃ to 1300℃ while it decreases at 1400℃, which may be due to crystal grain coarsening. So the sintering temperature of Ni-NiFe2O4 cermets in current work should be controlled at 1300℃, where the relative density is 96.85%.
Key words: Ni-NiFe2O4 cermets; densification; inert anode; aluminum electrolysis CLC
number: TF821 Document code: A
1 INTRODUCTION
Aluminum electrolysis consumes enormous energy and resources, and the consumption will be considerably decreased by introduction of inert anode and wettable cathode. However, under the tough working condition of aluminum electrolysis, i.e. highly corrosive Na3AlF6-Al2O3 molten salts at high temperature(940-960℃), the inert anodes necessary for the successful production of aluminum might have the following properties: be insoluble in a fluoride melt, be resistant towards anode oxygen, have good electrical conductivity, possess adequate strength at high temperature and be resistant to thermal shock and creep rupture[1, 2].
Cermets and self-passivating alloy are two kinds of candidate inert anode materials, which are intensively studied and seem to be most prospective. The so-called cermets are supposed to contain the advantages of both ceramic materials, i.e. low corrosion and oxidation, and metallic materials, i.e. good electrical conductivity and high thermal shock resistance. The typical cermets compose spinel oxide and metal, i.e. ceramic phases of NixFe3-xO4, NiyFe1-yO, NiFe2O4+NiO, ZnFe2O4+ZnO, or NixFe2yZnzO(3y+x+z±δ), and metal phases of Cu, Ni, Cu-Ni, Ni-Fe, Cu-Cr, or Cu-Ag. The densification of cermets is of great importance, because high relative density will reduce corrosion rate, lower electronic resistance, improve mechanical strength, avoid oxidation of metal phase and creep rupture because of electrolyte penetration[2-4]. Therefore, based on our previous work to avoid metal bleeding or cluster, in this paper, the effects of ball milling time of raw powder of Ni and NiFe2O4, contents of metal Ni, sintering atmosphere and sintering temperature on the density of Ni-NiFe2O4 cermets were studied and the relevant mechanisms were also discussed.
2 EXPERIMENTAL
Raw materials, nickel powder, NiO and Fe2O3 were all reagent grade. Ni-NiFe2O4 cermet samples were prepared with a conventional cold pressing-sintering technique. Proper amount of NiO and Fe2O3 were mixed by ball milling and calcined at 1150℃ for 6h under air to get the stoichiometric NiFe2O4 powder[5]. The NiFe2O4 powders and Ni were mixed and micronized by ball milling in the mediums containing dispersant and adhesive in planet ball mill. Finally the dried powders were pressed into cylindrical shape specimens(d20mm×40mm) at the biaxial pressure of 200MPa and sintered at 1100-1400℃ for 4h in controlled atmosphere to get the desired cermet samples[6].
Particle size distribution analysis was conducted with a laser particle size analyzer(LS601, Zhuhai, China). The relative density, porosity of the cermet samples were tested with the ASTM Archimedes Method C373-88(1999). Phase composition examination was performed with X-ray diffraction analysis in a Rigaku 3014 X-ray diffractometer. Microstructure and composition analysis were carried out using an XJP-6A optical microscope and a scanning electron microscope(JSM-5600LV).
3 RESULTS AND DISCUSSION
3.1 Effect of ball milling time for raw powders of Ni and NiFe2O4
In the current work the effects of different ball milling time ranging from 0 to 600min, on the mean particle size and the relative densities were studied. The sintering was conducted at 1200℃ for 4h and the rotating rate of ball milling was kept at 240r/min. The results are listed in Table 1.
As listed in Table 1, the relative densities of green samples and sintered samples increase with prolonging ball milling time when the time is less than 150min; and the trend is on the contrary when the time is more than 150min. This indicates that the powder activity affecting the pressing and sintering densification, can be enhanced to some extent by prolonging ball milling time. The powder activity includes powder surface activity and lattice activity. The former depends on powder size and shape and the latter depends on lattice size, lattice defects, internal stress, and so on. The longer ball milling time, on one hand, makes the powder shape more irregular, the grain smaller, rusulting in increase of both activities; and on the other hand, brings about more lattice defects, enhancing sintering driving force, accelerating sintering densification[5-8]. After 150min, however, the mixed powders begin to assemble and become large, decreasing their activity. Though the sample green density increases because of granulation effect, the sintering density decreases. Therefore, the optimum ball milling time is 150min for Ni-NiFe2O4 in current work.
3.2 Effect of metal contents
Nickel is a good sintering aid for many compounds in sintering process[6]. Former studies[9-11] have found that relative densities of Ni-NiFe2O4 are higher than those of Cu-NiFe2O4 in the same condition, and much higher than those of NiFe2O4 sintered in air. So we deduce that nickel probably is beneficial to NiFe2O4 sintering. In this work, Ni-NiFe2O4 cermets with different Ni contents were sintered at 1200℃ for 4h in weak reductive atmosphere to investigate the effect of contents of Ni on Ni-NiFe2O4 sintering densification.
As listed in Table 2, the relative densities of Ni-NiFe2O4 cermets are constantly high with the Ni content varying in 0-15%(mass fraction). Compared with stoichiometric NiFe2O4 ceramics, relative densities of Ni-NiFe2O4 cermets are not improved. This may lie in two reasons. First, metal nickel has no function as sintering aids during Ni-NiFe2O4 cermet sintering. Second, its effect is probably the same as that of weak reductive atmosphere, i.e. they both bring about oxygen deficiencies, which can speed atomic diffusion and strengthen the sintering process, so the effect as sintering aids of sintering atmosphere probably overshadows that of Ni[12-14]. The effect of sintering atmosphere will be further discussed.
Also from Table 2, rather than what we have expected, when metal content is more than 5%(mass fraction), with the Ni content increasing, the relative densities slightly decreases. Although nickel is compatible with NiFe2O4 matrix, excessive Ni powder may lead to two bad effects. First, big metal grains form in 15%Ni-NiFe2O4 as shown in Fig.1, which causes steric hindrance and retards the samples densification when they locate at the boundary of NiFe2O4 grains[12, 13]. Second, they induce too many oxygen deficiencies which assemble to pores, increasing pores in crystals, so impairing Ni-NiFe2O4 densification[15].
Table 1 Change of physical properties of cermets with ball milling time
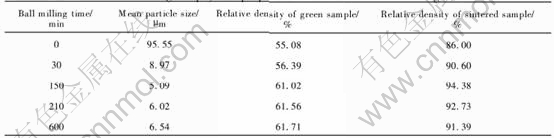
Table 2 Effect of Ni content on relative density of Ni-NiFe2O4 cermets
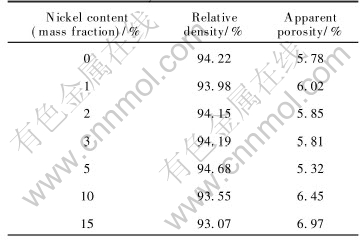
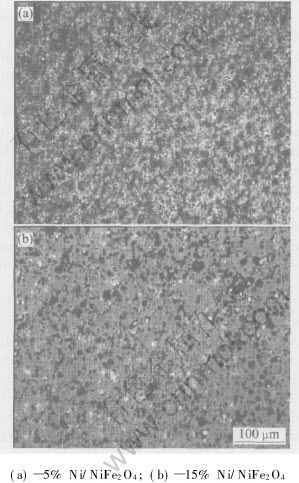
Fig.1 Microstructures of Ni-NiFe2O4 cermets
3.3 Effect of sintering atmosphere
Previous works[9- 11] investigated the controlling of sintering atmosphere, and its effect on the micro-composition of the cermets. In order to eliminate the effect of metal phase on the micro-composition and the densification process and to guide the densification of NiFe2O4 based cermets, the current work studies and compares the relative densities of stoichiometric NiFe2O4 ceramic sintered in different atmosphere. The results are summarized in Table 3.
In Table 3, the oxygen partial pressures of weak reductive atmosphere A and B are lower than the equilibrium oxygen pressure of the oxidation reaction for Ni or the decomposition reduction for NiO , and the formers is lower than that of latter. As listed in Table 3, the relative density of NiFe2O4 ceramic sintered in weak reductive atmosphere is 15% greater than that of NiFe2O4 ceramic sintered in air at 1200℃, and is close to that of NiFe2O4 ceramic sintered in air at 1400℃. NiFe2O4 is a kind of dislocable deficient compounds, so the larger relative density might result from the fact that weak reductive atmosphere can introduce oxygen deficiencies, which brings about lattice distortion, activation of sintering and increase of ceramic densification[14, 16, 17].
Table 3 Effect of sintering atmosphere on relative density of NiFe2O4
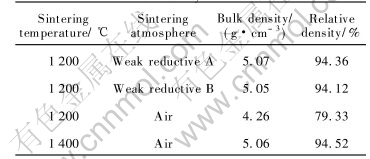
Fig.2 shows the X-ray diffraction patterns of NiFe2O4 sintered in weak reductive atmosphere A, B, and in air, and the former two are very similar to the last one, except for several FeNi3 peaks. Semi-quantitative analysis shows that the samples sintered in weak reductive A and B contain approximately 4% and 1%FeNi3(mass fraction), respectively, resulting from oxygen deficiencies increase induced by the weak reductive atmosphere. Because little FeNi3 phase hardly influences the corrosive resistance of cermets, eligible cermets inert anodes can be sintered in weak reductive atmosphere to get large densification.
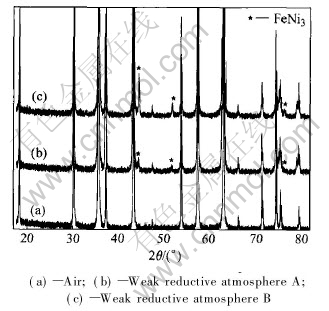
Fig.2 XRD patterns of NiFe2O4 ceramics sintered in different atmospheres
3.4 Effect of sintering temperature
Sintering temperature schedule is crucial to the densification of cermets. Previous works[10, 11] showed that nickel effuses from the cermets when sintered at the temperature above the melting point of Ni, i.e. 1453℃, which is harmful to samples properties such as electric conductivity and corrosion resistance. In present study, 5%Ni-NiFe2O4 were sintered at different temperatures below Nickels melting point, and held for the same period of 4h in weak reductive atmosphere.
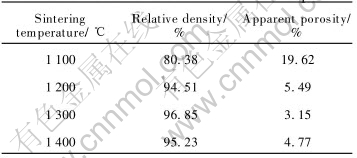
As summarized in Table 4, when the sintering temperature increases from 1100℃ to 1200℃, the relative density increases greatly from 80.38% to 94.51%, and the apparent porosity correspondingly decreases from 19.62% to 5.49%. Further increase in sintering temperature has little effect on the densification and sintering temperature higher than 1300℃, i.e. 1400℃; on the contrary, leads to lower relative density.
Table 4 Effect of sintering temperature on relative densities of 5%Ni-NiFe2O4 samples
In the solid-state sintering, Ni-NiFe2O4 sintering process at high temperature can be described as following steps. Firstly, in the relatively low temperature stage, with the sintering temperature increasing, sintering necks shrink, distance between grains is shortened, grains grow, open pores disappear and green samples shrink, which results in the increase of density and strength. Higher temperature can considerably accelerate this process, therefore, cermets density remarkably increases with the sintering temperature increasing. Secondly, when the relative density reaches about 90%, most of open pores become close; sintering process comes into the stage of close pore spheroidization, shrinkage, or disappearance. Increasing temperature can also increase the activation energies of material transport, strengthen sintering driving force, reduce pore surface and accelerate pore shrinkage and disappearance, but the effects on densification in this stage are much less than those in the first one[7, 8]. When the relative density is higher than 95%, temperature increasing might bring about anti-densification, as listed in Table 4, resulting in the phenomenon that the relative density of the sample sintered at 1400℃ is lower than that of samples sintered at 1300℃.
There might be several reasons leading to the anti-densification process. Firstly, the gas is insolvable to the cermets, thus the gas pressure in close pores quickly rises with the temperature increasing, and the shrinkage stops when the pressure is greater than the pore surface tension force. Secondly, higher temperature leads to further particle growth, which is detrimental to the densification process. Fig.3 shows that the grain size of sample sintered at 1200℃ is about 10μm; whereas that of samples sintered at 1400℃ is about 15μm. Thirdly, too higher temperature induces Kirkendall effect of material(atom, ion, or deficiency), which has adverse impact on densification[6, 7].

Fig.3 SEM images of 5%Ni-NiFe2O4 sintered at different temperatures
Therefore the optimum sintering temperature for Ni-NiFe2O4 cermets is 1300℃, resulting in the highest relative density. From the analysis above, the temperature at first stage of sintering should be elevated slowly to decelerate the early densification and reduce the formation of close pores. And the sintering temperature should not be too high; the holding time should not be too long.
4 CONCLUSIONS
1) At the rotating rate of 240r/min under present condition, the proper ball milling time is 150min, with which the mean diameter of mixed Ni-NiFe2O4 powders is 5.09μm and the density of sintered cermets is 94.38%.
2) Cermets containing 0-15%Ni have high relative density ranging from 94% to 96%, but with the Ni content increasing, the density slightly decreases because of metallic grains growth. Nickel powders probably have no function as sintering aids or this function is concealed by the effect of weak reductive sintering atmosphere.
3) Sintering atmosphere is vital to densification of Ni-NiFe2O4 cermets. Weak reductive sintering atmosphere induces more oxygen deficiencies which reinforce the sintering process and accelerate the densification.
4) Sintering temperature greatly influences densification of Ni-NiFe2O4 cermets. Properly high temperature is of benefit to densification. The relative density of Ni-NiFe2O4 cermets increases from 80.38% to 96.85% with the sintering temperature increasing from 1100℃ to 1300℃; while it decreases at 1400℃, which may be due to crystal grain coarsening. Furthermore, too high temperature also brings about irregular lattice growth, and the mean grain size of cermets sintered at 1200℃ is about 10μm, whereas that of sample sintered at 1400℃ is about 15μm.
REFERENCES
[1]Pawlek R P. Inert anodes: an update [A]. Wolfgang Schneider. Light Metals 2002 [C]. Warrendale PA, USA: TMS, 2002. 449-456.
[2]Sadoway D R. Inert anodes for the Hall-Héroult cell: the ultimate materials challenge [J]. JOM, 2001, 53(5): 34-35.
[3]LIU Ye-xiang. Progress of investigation and development on inert anodes and wettable cathodes in aluminum electrolysis [J]. Light Metals, 2001(5): 26-29.( in Chinese)
[4]Pawlek R P. Inert anodes for the primary aluminum industry: an update [A]. Hale W R, Light Metals 1996 [C]. Warrendale PA, USA: TMS, 1996. 243-248.
[5]XIONG Wei-hao, ZHOU Feng-yun, LI Guo-an, et al. Influence of powder particle size on the structure and properties of Ti(C, N)-based cermets [J]. J Huazhong Univ of Sci & Tech, 1995, 23(13): 37-41.
[6]GUO Shi-ju. Theory of Powder Sintering [M]. Beijing: Metallurgical Industry Press, 1998.
[7]HUANG Pei-yun. Powder Metallurgy Principle [M]. Beijing: Metallurgical Industry Press, 1997.
[8]YE Rui-lun, FANG Yong-han, LU Pei-wen. Physical Chemistry of Inorganic Materials [M]. Beijing: China Architecture & Building Press, 1984.
[9]QIN Qing-wei, LAI Yan-qing, ZHANG Gang, et al. Solid state reaction synthesis of Ni(1-x)ZnxFe2O4 spinel used as matrix of inert anodes in aluminum electrolysis [J]. The Chinese Journal of Nonferrous Metals, 2003, 13(3): 769-773.(in Chinese)
[10]ZHANG Gang, LAI Yan-qing. Preparation of nickel ferrite based cermets for aluminum electrolysis [J]. Journal of Material Science and Engineering, 2003, 21(4): 44-47.
[11]TIAN Zhong-liang, LAI Yan-qing, ZHANG Gang, et al. Preparation of NiFe2O4-Cu based cermet inert anodes in aluminum electrolysis [J]. The Chinese Journal of Nonferrous Metals, 2003,13(6): 1540-1545.
[12]XU Ming-xia, DUAN Ren-guan. Effect of Al2O3 content on the sinterability and mechanical properties of Y-TZP ceramics [J]. Bulletin of the Chinese Ceramic Society, 1997, 4: 40-42.
[13]WANG Xin, WANG Pei-ling, CHENG Yi-bing. Effect of TiO2 and MgO additions on microstructures of Al2O3 [J]. Journal of Inorganic Materials, 2001, 16(5): 979-984.
[14]GU Feng, SHEN Yue, GUO Min, et al. The effect of sintering atmosphere on the mechanical properties of 95Al2O3 ceramics [J]. Journal of Shanghai University (Natural Science), 2000, 6(5): 396-398.
[15]WAN Jun-xi, ZENG Qing-guang, XU Yu-fen. Influence of rare-earth oxide on the densification of pressureless sintering zirconia ceramics [J]. Journal of Hefei University of Technology, 2000, 23(4): 555-557.
[16]LIU Wei-yue, LIU Xiong-guang. The influence of sintering atmosphere on densification of ZTM/SiC composite [J]. Journal of Shanghai University, 1996, 29(5): 721-726.
[17]CUI Guo-wen. Deficiencies, Diffusion and Sintering [M]. Beijing: Tsinghua University Press, 1990.
(Edited by YANG Bing)
Foundation item: Project(G1999064903) supported by the National Basic Research Program of China; Project(2001AA335013) supported by Hi-tech Research and Development Program of China
Received date: 2004-10-22; Accepted date: 2005-01-05
Correspondence: LAI Yan-qing, Associate professor, PhD; Tel: +86-731-8876454; E-mail: iline@mail.csu.edu.cn