DOI: 10.11817/j.ysxb.1004.0609.2020-39554
二氧化锰矿分解连二硫酸锰及动力学
丁 林,杨 林,王 成,姚 露,蒋文举,江 霞
(四川大学 建筑与环境学院 国家烟气脱硫工程技术研究中心,成都 610065)
摘 要:氧化锰矿脱硫是一种新型大气污染控制协同硫资源化脱硫技术,但脱硫过程中除生成MnSO4外,还会生成副产物MnS2O6,影响脱硫浆液的资源化利用。本文以MnO2矿为原料,研究了脱硫锰浆中低浓度MnS2O6的强化分解过程,讨论了MnS2O6浓度、反应时间、反应温度、硫酸浓度和锰矿投加量等关键反应条件对MnS2O6分解的影响。并对MnO2矿强化氧化分解MnS2O6进行了动力学分析。结果表明:MnO2矿强化氧化分解MnS2O6的表观反应级数为2,反应活化能为74.8 kJ/mol,属于化学反应控制过程。
关键词:锰矿脱硫;连二硫酸锰(MnS2O6);MnO2矿;氧化分解;动力学
文章编号:1004-0609(2020)-10-2475-07 中图分类号:X506;TQ031.7 文献标志码:A
近年来,基于大气污染控制的氧化锰矿脱硫的硫资源化技术得到了快速发展[1-4],它主要是利用烟气中SO2与MnO2通过氧化还原反应生成MnSO4,实现烟气中SO2的脱除,同时,实现锰矿粉中锰组分的浸出,反应过程如式(1)和(2)所示。然而,氧化锰矿脱硫反应过程将产生一种无法避免的副产物连二硫酸锰(MnS2O6)(见式(3))。
MnO2+SO2→MnSO4 (1)
SO2+0.5O2+H2O→H2SO4 (2)
MnO2+2SO2→MnS2O6 (3)
脱硫浆液中MnS2O6的存在对脱硫液的资源化利用具有巨大的影响,如生产硫酸锰产品时易共结晶进入产品,导致产品纯度降低、表观色度变差和易结块等问题。而将其用于生产电解金属锰时,MnS2O6的存在对电解上板具有抑制作用,研究表明电解中性液中MnS2O6的浓度高于5 g/L时即无法实现电解上板[5-6]。因此,控制氧化锰矿脱硫液中MnS2O6浓度对脱硫浆液的资源化利用至关重要。
MnS2O6是脱硫过程中SO2的不完全氧化产物,具有一定的化学不稳定性,在强酸或高温条件下MnS2O6可以通过歧化反应分解为MnSO4和SO2:
MnS2O6→MnSO4+SO2 (4)
有研究发现[7],连二硫酸根也可被直接氧化生成硫酸(见式(5))。
MnS2O6+H2O+Oxidant→MnSO4+H2SO4 (5)
孙维义等[7]对软锰矿浆烟气同步脱硫脱硝尾液中连二硫酸锰分解特性进行了研究,结果表明,MnS2O6分解速率随H2SO4浓度、MnS2O6浓度和温度的升高而增大,随MnSO4浓度的增大而减小。但是,该研究所使用的是高初始浓度MnS2O6(约34 g/L)脱硫液。而对于MnS2O6初始浓度较低(≤10 g/L)情况下如何加快MnS2O6氧化分解并实现其深度去除的研究还鲜见报道。
MnO2本身即是一种优异的氧化剂,如果将天然MnO2用于高效氧化去除MnS2O6,将极大地降低技术成本。然而,目前关于MnO2,特别是关于含MnO2矿消除MnS2O6的研究较少[8-9],有关动力学的研究仍旧匮乏。因此,本研究以天然MnO2矿为氧化剂,系统研究了MnS2O6不同初始浓度、反应温度、反应时间、硫酸浓度和矿粉投加量等工艺参数对MnO2氧化分解MnS2O6性能的影响,并对该反应过程的反应动力学进行了全面分析,以期为氧化锰矿脱硫技术的工程化推广应用提供技术支持。
1 实验
1.1 主要材料、试剂和仪器
主要材料、试剂:含MnS2O6的锰矿浆脱硫浆液是由课题组自建的锰浆烟气脱硫中试系统制得。试验所用MnO2矿产自加蓬共和国,成分分析结果表明其全锰含量为40.35%,其锰组分主要以δ-MnO2形态存在。实验过程中用到的H2SO4、I2、Na2S2O3·5H2O、MnSO4·H2O和CH3COOH试剂均为分析纯,由成都科龙化学品有限公司生产,使用前不再做前处理。实验研究所用仪器设备主要有AUY120型分析天平、DL-101S型集热式恒温加热磁力搅拌器、DL-1型电子万用炉和G-100S型超声波清洗机。
1.2 实验方法
1.2.1 MnS2O6分解
自制脱硫液中MnS2O6初始浓度大于15000 mg/L。实验时通过稀释处理得到设定浓度的含MnS2O6脱硫液,为保证浆液性质统一,稀释后浆液通过添加MnSO4·H2O调节Mn2+浓度一致,稀硫酸用于调节浆液pH。
取50 mL含MnS2O6脱硫液置于100 mL锥形瓶中,然后加入一定量的硫酸,搅拌均匀后将锥形瓶迅速转移至磁力搅拌恒温水浴中。迅速加入指定量的MnO2矿然后密封,于恒温水浴、200 r/min磁力搅拌条件下反应。反应一定时间后,取适量反应液并过滤,测定滤液中MnS2O6的剩余浓度。MnS2O6分解率按式(6)所示进行计算:
 (6)
(6)
式中: 为MnS2O6的分解率(%);
为MnS2O6的分解率(%); 和
和 为MnS2O6的初始和剩余浓度(mg/L)。
为MnS2O6的初始和剩余浓度(mg/L)。
1.2.2 MnS2O6浓度测定
采用蒸馏-碘量法测定样品的MnS2O6浓度[10]。具体为:待测样品加硫酸后加热,样品中MnS2O6受热分解生成SO2。以N2为载气,碘液为吸收液,淀粉为指示剂,吸收样品分解产生的SO2。吸收液以Na2S2O3标准溶液滴定,同时做空白对照实验。MnS2O6的浓度计算如式(7)所示:
 (7)
(7)
式中: 为MnS2O6浓度(mg/L);V为主、副吸收瓶中碘标准体积(mL);
为MnS2O6浓度(mg/L);V为主、副吸收瓶中碘标准体积(mL);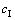 碘标准溶液浓度(mol/L);V0为空白样消耗硫代硫酸钠标准溶液(mL);V1为样品滴定硫代硫酸钠标准溶液体积(mL);c为硫代硫酸钠标准溶液浓度(mol/L);Vr为反应液取样体积(mL)。
碘标准溶液浓度(mol/L);V0为空白样消耗硫代硫酸钠标准溶液(mL);V1为样品滴定硫代硫酸钠标准溶液体积(mL);c为硫代硫酸钠标准溶液浓度(mol/L);Vr为反应液取样体积(mL)。
2 结果与讨论
2.1 MnO2矿分解MnS2O6工艺研究
2.1.1 初始浓度对MnS2O6分解的影响
配制初始浓度分别为4000、6000、8000、10000和12000 mg/L的含MnS2O6脱硫溶液,分别取50 mL于100 mL锥形瓶,然后依次加入10.0 g的MnO2矿,硫酸浓度为1.4 mol/L,于90 ℃恒温条件下反应。不同MnS2O6初始浓度下MnS2O6剩余浓度随时间的变化如图1所示。

图1 不同初始浓度下MnS2O6浓度随时间的变化情况
Fig. 1 MnS2O6 concentration varied with reaction time under different initial concentrations of MnS2O6
由图1可知,在不同初始浓度下,MnS2O6表现出相近的分解动力学行为,在初始的30 min内反应速率较快,MnS2O6浓度迅速降低,然后随着反应时间的延长逐渐减缓。这是因为随着反应过程的持续,溶液中H+的消耗会影响锰矿表面的电负性[11],MnS2O6的氧化分解速率减慢。随着MnS2O6初始浓度的增大,MnS2O6的分解效率逐渐降低,但MnS2O6的绝对氧化去除量逐渐升高。这是因为高浓度条件下反应的传质动力更大,更多的MnS2O6分子可以参与到分解反应中。而分解率的降低主要是由于在其他反应条件相同的条件下,初始反应物总量显著大于有效参与反应的部分。
2.1.2 硫酸浓度对MnS2O6分解的影响
配制MnS2O6初始浓度为8000 mg/L的含MnS2O6脱硫溶液,分别取50 mL于100 mL锥形瓶中并加入10.0 g的MnO2矿,然后分别加入1.0、1.2、1.4、1.6和1.8 mol/L的硫酸,于90 ℃恒温反应。不同硫酸浓度下MnS2O6浓度随时间的变化如图2所示。随着硫酸浓度的增大,MnS2O6的分解率逐渐升高,当硫酸浓度由1.0 mol/L提升到1.8 mol/L时,MnS2O6的分解率由56.7%升高至82.9%。这是因为MnO2氧化能力与脱硫液pH值密切相关,硫酸浓度增大使MnO2的表面氧化能力增强,进而强化了MnS2O6氧化分解[11-12]。同时,低pH反应条件下还能有效加速MnS2O6的自分解过程,多途径提高MnS2O6的氧化分解速率。
2.1.3 反应温度对MnS2O6分解的影响
配制MnS2O6初始浓度为8000 mg/L的含MnS2O6脱硫溶液,分别取50 mL于100 mL锥形瓶中,加入10.0 gMnO2矿和1.4 mol/L硫酸,于60、70、80、90和100 ℃恒温和磁力搅拌下反应。不同反应温度下MnS2O6剩余浓度随时间的变化如图3所示。由图3可知,随着反应温度的升高,MnS2O6的剩余浓度逐渐下降。当温度低于80 ℃时,MnS2O6的分解速率随反应温度的升高变化缓慢。当反应温度高于80 ℃时,MnS2O6分解速率快速升高。这是因为MnS2O6的分解是一个吸热反应,升高温度有助于加速MnS2O6的分解。此外,较高的反应温度也有助于强化MnO2氧化反应过程和反应活性[11]。两者共同作用实现MnS2O6分解效率的提升。

图2 不同硫酸浓度下MnS2O6浓度随时间的变化情况
Fig. 2 MnS2O6 concentration varied with reaction time under different H2SO4 contents

图3 不同温度下MnS2O6浓度随时间的变化情况
Fig. 3 MnS2O6 concentration varied with reaction time at different temperatures
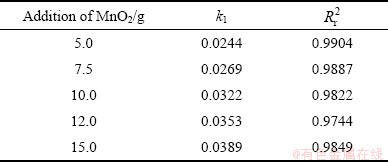
2.1.4 MnO2矿投加量对MnS2O6分解的影响
配制MnS2O6初始浓度为8000 mg/L的含MnS2O6脱硫溶液,取50 mL置于100 mL锥形瓶中,分别加入0、5.0、7.5、10.0、12.5和15.0 g的MnO2矿,然后再加入1.8 mol/L的浓硫酸,于90 ℃恒温,磁力搅拌反应。不同MnO2矿投加量时MnS2O6的剩余浓度随时间的变化如图4所示。由图4可知,不投加MnO2矿时MnS2O6的分解率只有55.5%。随着MnO2矿投加量的增加,MnS2O6的分解率也呈升高趋势,但MnO2矿的投加量对MnS2O6的分解作用影响较为微弱。当投加量高于10 g后,MnS2O6的分解率在80.0%基本保持不变。MnO2矿投加量的影响较小是由于其投加量本就远大于理论需要量,此时MnS2O6的分解过程为化学反应控制,因此,增加MnO2矿的投加量对MnS2O6的影响较为微弱。

图4 不同MnO2矿投加量MnS2O6浓度随时间的变化
Fig. 4 MnS2O6 concentration varied with reaction time under different MnO2 ore dosages
2.2 MnS2O6分解动力学研究
2.2.1 动力学模型
反应动力学描述了反应过程中反应物的反应速率,这一速率决定了工程应用时反应装置的停留时间和设备尺寸,对于大规模建设具有重要的实际意义。为更好地了解MnO2矿分解MnS2O6反应机理以及MnS2O6初始浓度、硫酸浓度、MnO2矿投加量和温度等因素对反应速率的影响,反应动力学研究至关重要。
MnO2矿分解MnS2O6的反应速率方程可以用指数经验模型表示:
 (8)
(8)
式中:t为反应时间(min); 为MnS2O6的浓度(mg/L);k1为反应速率常数;n为MnS2O6的表现反应级数。
为MnS2O6的浓度(mg/L);k1为反应速率常数;n为MnS2O6的表现反应级数。
由于MnS2O6的含量变化受到其他反应条件的影响,故式(8)可以改写为如式(9)的形式[13-14]:
 (9)
(9)
其中:
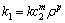 (10)
(10)
式中: 为硫酸浓度(mol/L);
为硫酸浓度(mol/L); 为MnO2的投加量(g/L);m为硫酸的表现反应级数;p为MnO2的表现反应级数。
为MnO2的投加量(g/L);m为硫酸的表现反应级数;p为MnO2的表现反应级数。
2.2.2 分解动力学研究

图5 不同MnS2O6浓度下MnS2O6的分解动力学
Fig. 5 MnS2O6 decomposition kinetics at different initial MnS2O6 concentrations
表1 不同MnS2O6初始浓度下MnS2O6分解动力学相关系数
Table 1 Kinetic correlation coefficient of MnS2O6 decomposition under different initial concentrations
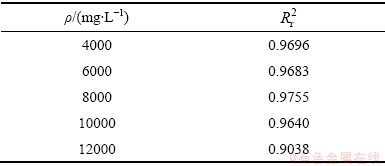
基于式(8),采用最小二乘法拟合处理图1所示数据,结果如图5所示,相关系数见表1。当n=2时,式(8)可以较好地描述不同MnS2O6初始浓度下MnS2O6分解情况, (
( 表示MnS2O6初始浓度)与t线性较好。将n=2代入式(9)积分可以得到
表示MnS2O6初始浓度)与t线性较好。将n=2代入式(9)积分可以得到
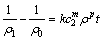 (11)
(11)
基于式(11)对不同硫酸浓度下MnS2O6的分解结果进行处理,结果如图6所示,速率常数和相关性系数见表2。不同硫酸浓度下, 与t具有较好的线性相关性,对式(10)两端取对数可得
与t具有较好的线性相关性,对式(10)两端取对数可得
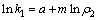 (12)
(12)
用式(12)对不同硫酸浓度下的速率常数k1进行处理,得出其关系式为
 (13)
(13)

图6 不同硫酸浓度下MnS2O6的分解动力学
Fig. 6 Decomposition kinetics of MnS2O6 at different H2SO4 contents
表2 不同硫酸浓度下MnS2O6分解动力学的速率常数和相关系数
Table 2 Rate constants and kinetics correlation coefficients of MnS2O6 decomposition under different acid contents

因此,得到连二硫酸锰分解反应动力学方程式中硫酸的表观反应级数为1.89。
对不同MnO2矿投加量实验结果按式(11)处理,结果如图7所示,速率常数和相关系数见表3。在不同MnO2矿投加下, 与t线性相关性较好,对式(10)取对数可得
与t线性相关性较好,对式(10)取对数可得
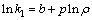 (14)
(14)
利用式(14)对不同MnO2矿投加量下的速率常数k1进行处理得到其关系式为
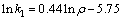 (15)
(15)
由此可得动力学方程式中MnO2矿的表观反应级数为0.44。

图7 不同MnO2矿投加量下MnS2O6的分解动力学
Fig. 7 Decomposition kinetics of MnS2O6 with different MnO2 ore dosages
表3 不同MnO2矿投加量下MnS2O6分解动力学的速率常数和相关系数
Table 3 Rate constants and correlation coefficients of decomposition kinetics of MnS2O6 under different MnO2 ore dosages
对不同温度下MnS2O6的分解结果按式(11)处理,其结果如图8所示,速率常数和相关系数见表4。不同温度下 与t线性相关性较好,速率常数k1为0.0016(60 ℃)、0.0030(70 ℃)、0.0042(80 ℃)、0.0143(90 ℃)、0.0278(100 ℃),根据阿伦尼乌斯方程式:
与t线性相关性较好,速率常数k1为0.0016(60 ℃)、0.0030(70 ℃)、0.0042(80 ℃)、0.0143(90 ℃)、0.0278(100 ℃),根据阿伦尼乌斯方程式:
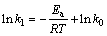 (16)
(16)
式中:Ea为反应表观活化能(kJ/mol);R为摩尔气体常数(8.314 J/(mol·K));T为反应温度(K)。
利用式(16)对不同温度下的速率常数k1进行处理得出其线性关系式为
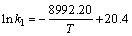 (17)
(17)
比较式(16)和式(17)可以求出,MnO2矿分解MnS2O6的反应活化能为74.8 kJ/mol。有研究证明,反应分子的扩散活化能小于20.0 kJ/mol,分子碰撞进行化学反应的活化能一般在40~400 kJ/mol之间[15],由此说明MnO2矿对MnS2O6的分解属于化学反应控制,温度升高会促进MnO2矿与MnS2O6的反应。

图8 不同温度下MnS2O6的分解动力学
Fig. 8 Decomposition kinetics of MnS2O6 at different temperatures
表4 不同温度下MnS2O6分解动力学的速率常数和相关系数
Table 4 Rate constants and kinetics correlation coefficients of MnS2O6 decomposition at different temperatures

3 结论
1) 在MnS2O6初始浓度较低条件下,提高反应温度、硫酸浓度以及一定范围内增加MnO2矿投加量,可加快MnS2O6的氧化分解,提高MnS2O6浓度分解率降低。
2) MnO2矿强化氧化分解中,MnS2O6的表现级数为2,反应活化能为74.8 kJ/mol,属于化学反应控制过程。工程实践中可通过调节液相的硫酸浓度、升温以及投加一定量的MnO2矿可以实现锰矿矿浆烟气脱硫中较低浓度MnS2O6的去除。
REFERENCES
[1] 朱晓帆, 蒋文举. 软锰矿脱除烟气中SO2的研究进展[J]. 中国锰业, 2001, 19(2): 10-12.
ZHU Xiao-fan, JIANG Wen-ju. Process and study on removal of SO2 in flue gas with pytolusite[J]. China’s Manganeses Industry, 2001, 19(2): 10-12.
[2] SUN Wei-yi, DING Sang-lan, ZENG Shan-shan, SU Shi-jun, JIANG Wen-ju. Simultaneous absorption of NOx and SO2 from flue gas with pyrolusite slurry combined with gas-phase oxidation of NO using ozone[J]. Journal of hazardous materials, 2011, 192(1): 124-130.
[3] SUN Wei-yi, SU Shi-jun, WANG Qing-yuan, DING Sang-lan. Lab-scale circulation process of electrolytic manganese production with low-grade pyrolusite leaching by SO2[J]. Hydrometallurgy, 2013, 133: 118-125.
[4] QU Bing, HU Wen-li, DENG Lin, SUN Wei-yi, DING Sang-lan, GAN Zhi-wei, SU Shi-jun. Simultaneous determination of dithionate and sulfate in leaching solution from SO2-leaching pyrolusite by ion chromatography[J]. Energy &Fuels, 2016, 30(10): 8561-8566.
[5] WARD C B. Hydrometallurgical processing of manganese containing materials: The United States, US7951282[P]. 2011-05-19.
[6] YOU Zhi-xiong, LI Guang-hui, ZHANG Yuan-bo, PENG Zhi-wei, JIANG Tao. Extraction of manganese from iron rich MnO2 ores via selective sulfation roasting with SO2 followed by water leaching[J]. Hydrometallurgy, 2015, 156: 225-231.
[7] 孙维义, 苏仕军, 丁桑岚, 蒋文举, 徐 莹. 软锰矿浆烟气同步脱硫脱硝尾液中连二硫酸锰分解特性的研究[J]. 四川大学学报(工程科学版), 2011, 43(3):166-170.
SUN Wei-yi, SU Shi-jun, DING San-lan, JIANG Wen-ju, XU Ying. Study on the decomposition characteristics of MnS2O6 in the tail liquid of simultaneous desulfurization and denitrification of soft manganese pulp flue gas[J]. Journal of Sichuan University (Engineering Science), 2011, 43(3): 166-170.
[8] QU Bing, DENG Lin, DENG Biao, HE Ke-jie, LIAO Bing, SU Shi-jun. Oxidation kinetics of dithionate compound in the leaching process of manganese dioxide with manganese dithionate[J]. Reaction Kinetics, Mechanisms and Catalysis, 2018, 123(2): 743-755.
[9] 陈建伟, 童张法, 陈志传, 高大明. 粗MnSO4溶液中MnS2O6的去除条件研究[J]. 环境工程学报, 2011(1): 177-180.
CHEN Jian-wei, TONG Zhang-fa, CHEN Zhi-chuan, GAO Da-ming. Study on conditions for removing MnS2O6 from MnSO4 solution[J]. Chinese Journal of Environmental Engineering, 2011(1): 177-180.
[10] SOFFER N. The determination of dithionate, sulphite and sulphate in manganese leach liquors[J]. Analyst, 1961, 86(1029): 843-849.
[11] 宋垠先. 氧化型锰矿石氧化焦化废水实验研究[D]. 合肥: 合肥工业大学, 2007.
SONG Yin-xian. Study on oxidation of coking wastewater by manganese oxide mineral[D]. Hefei: Hefei University of Technology, 2007.
[12] FURLANI, PAGNANELLI. Reductive acid leaching of manganese dioxide with glucose: Identification of oxidation derivatives of glucose[J]. Hydrometallurgy, 2006, 81(3): 234-240.
[13] 王雨红, 雷作敏, 屈欣轲, 黄丽滢, 侯佳敏, 欧阳秋林, 粟海锋. 葡萄糖在氧化锰矿浸出过程中的分解动力学[J]. 中国有色金属学报, 2016, 26(6): 1303-1310.
WANG Yu-hong, LEI Zuo-min, QU Xin-ke, HUANG Li-ying, HOU Jia-min, OUYANG Qiu-lin, LI Hai-feng. Oxidative breakdown kinetics of glucose in process of leaching manganese oxide ore[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(6): 1303-1310.
[14] 崔勍焱. 软锰矿氧化降解焦糖色素反应过程的研究[D]. 南宁: 广西大学, 2009.
CUI Qing-yan. Study on reaction process of caramel oxidative degradation using pyrolusite as a oxidant[D]. Nanning: Guangxi University, 2009.
[15] 林智信, 安从俊, 刘 义. 物理化学:动力学·电化学·表面及胶体化学[M]. 武汉: 武汉大学出版社, 2003.
LIN Zhi-xin, AN Cong-jun, LIU Yi. Physical chemistry: Kinetics, electrochemistry, surface and colloidal chemistry[M]. Wuhan: Wuhan University Press, 2003.
Decomposition of manganous dithionate with natural MnO2 ore and kinetics study
DING Lin, YANG Lin, WANG Cheng, YAO Lu, JIANG Wen-ju, JIANG Xia
(College of Architecture and Environment, National Engineering Research Center for Flue Gas Desulfurization, Sichuan University, Chengdu 610065, China)
Abstract: Manganese oxide desulfurization is a new desulfurization technology and coupled with the resource utilization of sulfur simultaneous. However, the desulfurization process has an inevitable byproduct, the manganese dithionate (MnS2O6), which affect the following resource utilization. In this study, the natural manganese oxide ore (δ-MnO2) strengthened low concentration MnS2O6 decomposition was carefully studied. The effects of operation conditions, including the original MnS2O6 concentration, reaction time, temperature, slurry acidity and manganese ore addition on decomposition of MnS2O6 were discussed, and the kinetics was also analyzed based on these results. The results show that the apparent reaction order of MnS2O6 with natural MnO2 ore is 2, and the activation energy is 74.8 kJ/mol, which is a typically chemical reaction control process.
Key words: manganese oxide ore desulfurization; manganese dithionate (MnS2O6); natural MnO2 ore; oxidation decomposition; kinetics
Foundation item: Project(2018YFC0213405) supported by the National Key Research and Development Program of China
Received date: 2019-09-20; Accepted date: 2020-07-20
Corresponding author: YANG Lin; Tel: +86-28-85403016; E-mail: andyyiyin@scu.edu.cn, evanlinyang@sina.com
(编辑 龙怀中)
基金项目:国家重点研发计划资助项目(2018YFC0213405)
收稿日期:2019-09-20;修订日期:2020-07-20
通信作者:杨 林,副研究员,博士;电话:028-85403016;E-mail:andyyiyin@scu.edu.cn, evanlinyang@sina.com