
Synthesis and properties of nanocrystalline nonferrous metals prepared by flow-levitation-molding method
LIU Wei(刘 伟)1, YANG Tian-zu(杨天足)1, CHU Guang(楚 广)1, 2, LUO Jiang-shan(罗江山)2, TANG Yong-jian(唐永建)2
1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. Institute of Laser Fusion, China Academy of Engineering Physics, Mianyang 621900, China
Received 15 July 2007; accepted 10 September 2007
Abstract: Nanocrystalline nonferrous metals (Cu, Al, and Ag) were synthesized by flow-levitation-molding method. The microstructure of the as-prepared nanocrystalline metals was characterized by XRD and FESEM. The microhardness and electrical resistivity were tested by the HMV-2 type Microhardness Tester and 6157 type Electrometer, respectively. The synthesis process was also studied. The results show that the spheriform particles in nanocrystalline metals have average grain size of 20-30 nm. The relative density of nanocrystalline Cu, Al, and Ag are 95.1%, 98.1% and 98.3%, respectively. The microhardness of nanocrystalline Cu, Al and Ag are 2.01, 2.11 and 1.26 GPa respectively, which are larger than those of their coarse-grained counterparts by the factor of 4.5, 14, and 2.5, respectively. The electrical resistivity of nanocrystalline Cu at room temperature is 1.5×10-7 Ω·m, which is higher than coarse-grained Cu by a factor of 7.5. The pressure is the predominant factor influencing the density of the as-prepared nanocrystalline nonferrous metals.
Key words: nanocrystalline; nonferrous metals; flow-levitation-molding; density; microhardness; electrical resistivity
1 Introduction
Nanocrystalline (Nc-) materials are three dimensional solid materials that are composed of nano-sized grains or crystallites. Compared with the coarse-grained (cg) materials, the average grain size of nc-materials is so small (<100 nm) that a large number of atoms are existing at the grain boundary, and the proportion of grain boundary is up to 50%. Grain boundary is an important component of materials, not just considered a defect. Owing to their unique structure, nc-materials have many remarkable properties, such as high microhardness[1-2], high strength[3-4], low temperature superplasticity[5] and grain softening phenomenon[6-8]. Researches on nc-materials can widen the comprehension of the lattice defects theory and the intrinsic relationship between the property and crystal structure.
In recent years, there has been growing interest in synthesis of nc-metals for the nc-metals can be widely used only when they can be prepared by easy and efficient methods. At present, there are two main approaches to synthesize nc-materials. One is “from powder to bulk”, that is, the metal powder is prepared in advance and then compressed to synthesize bulk nc-material. It includes inert gas condense in-situ compaction(IGC)[1,4], mechanical alloying(MA)[9], electrical deposition(ED)[5], and so on. The other approach is the so called “from coarse-grain to nanocrystalline”, that is, the coarse-grained bulk materials can transform to nc-material through particular processes. It includes severe plastic deformation(SPD) [10-11], amorphous alloy crystallization method[12], and so on. However, all these methods have some limitations. For example, the yield and the purity of the particle surface are the restricting factors for IGC and MA. Nc-materials synthesized by SPD are fully dense and without any pores or contaminant. But the average grain size of the as-prepared specimens is about 100 nm. So it cannot embody the particular properties of the nc-materials with the average grain size below 50 nm.
In this work, we aim to synthesize nanocrystalline nonferrous metals (Cu, Al, and Ag) by a new method:flow-levitation-molding method(FLM) and test some of their properties. Compared with nano-particles prepared by IGC and MA, nano-particles prepared by flow- levitation method(FL)[13] have high purity for the heating system is non-touching. The average grain size of nano-particle prepared by LF technology can be controlled easily through adjustment of process parameters. For these reasons, FLM technology is favor of the industrialization of nano-technology.
In this work, the compaction parameters were studied carefully to identify their influence upon the density of the as-prepared nc-metals. The microhardness and electrical resistivity of the as-prepared nc-metals were tested. Also, the micro-structure of these nc-nonferrous metals was characterized by X-ray diffraction(XRD) and field emission scanning electronic microscopy(FESEM).
2 Experimental
Nano-sized Cu, Al, and Ag particles were synthesized by flow-levitation method and then filled into the high strength mold at ZKSTX-1 type vacuum- gloves-chest with high purity Ar atmosphere (purity>99.99%) protection. Subsequently, the mold was put into the chamber of TDY15-50T type hydraulic press. Afterward, under a uniaxial load of 1.0-1.75 GPa for duration of 2-60 min, the nono-particles were compacted into disc-shape samples with 10 mm in diameter and 0.5-1.5 mm in thickness.
Philips X’Pert Pro MPD type X-ray diffraction (made in Netherlands, Cu target, Kα radiation, λ= 0.154 056 nm) was used to identify the phase of the as-prepared specimens. The average grain size was measured according to XRD patterns with Warren- Averbach Fourier transfer method(W-AFT)[14] of (111), (200), (220) peaks. The Archimedes’ principle was used to measure the density. The Vickers microhardness measurements (HMV-2, standard error is 0.2%) were taken ten times on each specimen using 19.6 N load applied for 10 s. All the specimens were polished and inlayed into the epoxy resin before the microhardness measurement. Keithley model 6157 electrometer (made in USA) was used to measure the electrical resistivity of the as-prepared nc-Cu at 233-293 K. Sirion200 FESEM was employed to observe the inner structure of the as-prepared specimens.
3 Results and discussion
3.1 Analysis of compaction process
In this work, the as-prepared nc-nonferrous metals (Cu, Al, and Ag) have smooth surfaces without cracks and rough edge. The relative density of nc-Cu, nc-Al and nc-Ag are 95.1%, 98.1% and 98.3%, respectively.
Fig.1 and Fig.2 show the relationship curves of relative-density vs pressure and relative-density vs pressure-holding-time, respectively. It is found that the pressure and pressure duration have significant impact upon the density of the as-prepared specimens during compaction.
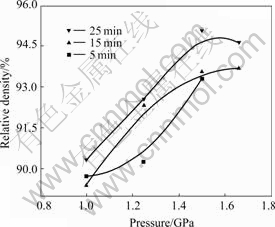
Fig.1 Pressure—relative density curves with different pressure holding times

Fig.2 Pressure holding time—relative density curves at different pressures
As shown in Fig.1, at the beginning of the compaction process, the increase of density is obvious with the increasing pressure. At this stage, densification is driven by the motion of particles and the filling of void among particles. When the pressure continues increasing, the nano-particles deform. Take Cu for example, the nano-Cu particles in this work deform when the pressure is above 0.6 GPa according to the calculated results taken by NITH et al[15]. The density increases insignificantly when the pressure is above 1.5 GPa. This is because: 1) the contact area between nano-particles is increasing during densification, the strength of the material adjoining the contact area is increased, too; 2) the friction between particles is very large because the grain size of the nano-particles is smaller than coarse-grained materials. In this work, the highest pressure is 1.75 GPa for the highest strength of the mold restrict the elevation of the pressure.
Fig.2 also shows that the pressure holding time has little influence on the density of the as-prepared nc-nonferrous metals when the pressure is relatively low (below 1 GPa). However, the density of the nc-specimens increases slightly with longer pressure holding time when the pressure is above 1.25 GPa. Our interpretation of these results is as follows: the pressure is very high in the experiment, when the pressure holding time is too short, the volume of the specimen expands because of the huge elastic aftereffect. So during the experimental, it is necessary to prolong the pressure holding time. Then the internal stress can be expected to release fully during the pressure holding process.
3.2 Average grain size and structure characterization
As we know, the nano-sized grains have large surface energy and are at metastable state. According to the XRD spectra of nano-Cu particles and nc-Cu in Fig.3, the nc-Cu specimen prepared by FLM method is FCC (face centered cubic) structure, without being oxidized. This is because the loading and compaction process are under inert gas protection. Contrarily, the XRD spectra of nano-particle shows that the particles are oxidized a little due to the high surface activity of the particles (the specimens were exposed in air atmosphere during XRD tests).

Fig.3 X-ray diffraction patterns of Cu nanoparticles and nc-Cu
The average grain size of the specimens was calculated by the W-AFT method. Table 1 lists the calculated average grain size of the nc-nonferrous metals prepared at different compacting pressures (all these specimens were prepared with the same duration of 60 min).
The average grain size of the Cu particles before
Table 1 Average grain sizes of nanocrystalline nonferrous metals compacted under different pressures (nm)

compaction was 11.8 nm. Meanwhile, the average grain size of nc-Cu compacted at different pressures was about 20 nm. Different pressures did not change the average grain size of the nano-Cu much for the high pressure (GPa) restrained the grain growth. As far as nc-Al and nc-Ag concerned, compared with their nano-particles, the average grain size reduced with increasing pressure.
The changes of the average grain size of the as-prepared nc-metals may be related with the recrystallization process. During compaction, the heat generated from friction among nc-particles, the particles absorbed the heat and then began to recrystallize. The new nuclei of crystals began to form and grow in the nc-particles. As we know, the recrystallization temperature is related with the original grain size. The smaller the original grain size, the lower the recrystallization temperature. So, it is possible that during the compaction, the nano-particles of Cu accomplish the recrystallization process and the new nuclei begin to grow. While the nano-particles of Al and Ag do not complete the recrystallization process and the nuclei just form and do not grow significantly for the initiate recrystalline temperature is high due to the relative large grain size.
Figs.4-6 show the FESEM photographs of nc-Cu (prepared under pressure of 1.63 GPa), nc-Al and nc-Ag (prepared under pressure of 1.75 GPa), respectively. In these photographs, it can be found that the fine nano-particles deform and congregate under high pressure. The average particle size of the nc-Cu and nc-Al is about 30-80 nm. The average particle size of the nc-Ag is relatively large, it is about 500 nm. Although the consolidation is firm, there are still some micro-pores in these specimens.

Fig.4 FESEM photograph of nanocrystalline Cu
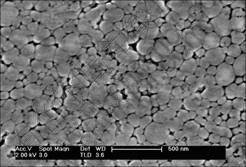
Fig.5 FESEM photograph of nanocrystalline Al

Fig.6 FESEM photograph of nanocrystalline Ag
3.3 Microhardness
The data of microhardness of the as-prepared nc-nonferrous metals are list in Table 2. There are also some data from other literatures. In this work, the microhardness of nc-Cu is 2.01 GPa, which is higher than coarse-grained Cu by a factor of 4.5. It is comparable with the date reported in Refs.[16-17].
The microhardness of nc-Al and nc-Ag is higher than their counterparts by the factor of 14 and 2.5 respectively. The high microhardness of the as-prepared nc-metals may be due to the fact that the specimens have small grained size, high density and fewer flaws. These data in Table 2 also indicate that the high microhardness is one of the intrinsical character of the nc-metals.
3.4 Electrical resistivity
GLEITER et al[20] have reported that the electrical resistivity of nc-metals is usually higher than their coarse-grained counterparts. There are large grain boundary regions in the nc-materials and the activities of electrons are restricted to be only around the small nano-crystallines. If the grain boundary is more thicker, the grains arrayed more disorderly and the electrons are scattered more strongly. Therefore the electrical resistivity
Table 2 Microhardness of nc-nonferrous metals

* cg is abbreviation of coarse-grained
of nc-metals is usually high. Fig.7 shows the temperature (243-293 K) dependence of the electrical resistivity of nc-Cu specimens prepared by FLM. The electrical resistivity of nc-Cu at room temperature is about 1.5×10-7 Ω·m, which is in a reasonable quality compared with the experimental results of LU et al[21]. The electrical resistivity is higher than the coarse-grained Cu (0.2×10-7 Ω·m) by a factor of 7. The electrical properties of other nc-nonferrous metals are under researching.
The relationship between the electrical resistivity and measuring temperature (243-293 K) fits the Matthissen equation[20]:
ρ=ρ0(1+αT) (1)
where ρ0 is the electrical resistivity at absolute zero, α is the temperature coefficient, T is the temperature. According to Fig.7, the calculated date of ρ0 and α are 4.64 μΩ·cm and 0.0043. The value of α is lower than the reported result in Ref.[21]. The low value of α indicates the electrical resistivity varies insignificantly when the temperature changes. This phenomenon may relate with
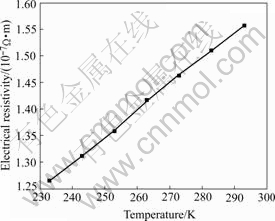
Fig.7 Dependence of electrical resistivity on temperature for as- prepared nc-Cu
the particular microstructure of nc-metals and it needs further research.
4 Conclusions
1) Nanocrystalline nonferrous metals (Cu, Al, and Ag) are synthesized by flow-levitation-molding method. The relative density of the nanocrystalline Cu, Al, and Ag are 95.1%, 98.1% and 98.3%, respectively. The average grain size of the as-prepared nc-metals ranges from 20 to 30 nm.
2) The pressure is the predominant factor influencing the density of the as-prepared nanocrystalline nonferrous metals. The density increases with higher pressure. The density increases insignificantly when the pressure-holding time is too long. But the specimen has better quality (less cracks, low elastic aftereffect, and so on) with proper pressure holding time.
3) The average grain size of nc-Cu grows after compaction while the average grain size of nc-Al and nc-Ag becomes smaller after compaction. This phenomenon is related with the recrystallization process probably.
4) The microhardness of nanocrystalline Cu, Al and Ag are 2.01, 2.11 and 1.26 GPa respectively, which are larger than their coarse-grained counterparts by the factor of 4.5, 14, and 2.5 respectively. High microhardness is the intrinsic property of the nc-metals.
5) The electrical resistivity of nanocrytalline Cu at room temperature is 1.5×10-7 Ω·m, which is higher than that of the coarse-grained Cu by a factor of 7.
References
[1] GLEITER H. Nanocrystalline materials [J]. Prog Mater Sci, 1989, 33: 223-315.
[2] CHEN J, LU L, LU K. Hardness and strain rate sensitivity of nanocrystalline Cu [J]. Scripta Mater, 2006, 54(11): 1913-1918.
[3] CHAMPION Y, GU?RIN-MAILLY S, BONNENTIEN J L, LANGLOIS P. Fabrication of bulk nanostructured materials from metallic nanopowders: Structure and mechanical behavior [J]. Scripta Mater, 2001, 44(8/9): 1609-1613.
[4] ZHOU Yu-song, WU Xi-jun, XU Guo-liang, LI Bing-han, ZHANG Hong-fei, DU Li-guang, LI Zong-quan. Synthesis, microdefects and mechanical properties of large bulk nanocrystalline silver and copper [J]. The Chinese Journal of Nonferrous Metals, 2000, 10(4): 465-469. (in Chinese)
[5] LU L, SUI M L, Lu K. Superplastic extensibility of nanocrystalline copper at room temperature [J]. Science, 2000, 287: 1463-1466.
[6] SCHIΦTZ J, FRANCESCO D DI T, KARSTEN W J. Softening of nanocrystalline metals at very small grain size [J]. Nature, 1998, 391: 561-563
[7] SWYGENHOVEN H V. Grain boundaries and dislocations [J]. Science, 2002, 296: 66-67.
[8] ANDREW J D, CHRISTOPHER A S. Tailoring and patterning the grain size of nanocrystalline alloys [J]. Acta Materialia, 2007, 55(1): 371-379.
[9] SHEN T D, GE W Q, WANG K Y, QUAN M X, WANG J T, WEI W D, KOCH C C. Structural disorder and phase transformation in graphite produced by ball milling [J]. Nanostruct Mater, 1996, 7(4): 393-399.
[10] VALIEVE R Z, ISLAMGALIEV R K, ALEXANDROV I V. Bulk nanostructured materials from severe plastic deformation [J]. Prog Mater Scie, 2000, 45: 104-189.
[11] MISHRA R S, MCFADDEN S X, MUKHERJEE A K. Analysis of tensile superplasticity in nanomaterials [J]. Mater Sci Forum, 1999, 304/306: 31-38.
[12] LU K, WEI W D, WANG J T. Microhardness and fracture properties of nanocrystalline Ni-P alloy [J]. Scripta Metal Mater, 1990, 24(12): 2319-2323.
[13] LI C M, LEI H, TANG Y J, LUO J S, LIU W, CHEN Z M Production of copper nanoparticles by the flow-levitation method [J]. Nanotechnology, 2004, 15: 1866-1869.
[14] GUO L H, LI H. Fabrication and characterization of thin nano- hydroxyapatite coatings on titanium [J]. Surf Coat Tech, 2004, 185(2/3): 268-274.
[15] NITH T G, WADSWORTH J. Hall-Petch relation in nanocrystalline solids [J]. Scripta Metall Mater, 1991, 25(4): 955-958.
[16] NIEMAN G W, WEERTMAN J R, SIEGE R W. Mechanical behavior of nanocrystalline Cu and Pd [J]. J Mater Res, 1991, 6(5): 1012-1027.
[17] CHOKSHI A H,RISEN A, KARCH J, GLEITER H. On the validity of the Hall-Petch relationship in nanocrystalline materials [J]. Scripta Metall, 1989, 23(10): 1679-1684
[18] CONG Hong-tao. Synthesis, characterize and mechanical properties of nano-Al-based component materials [D]. Shenyang: Institute of Metal Research, Chinese Academy of Sciences, 2003. (in Chinese)
[19] KIZUKA T, ICHINOSE H, ISHIDA H J. Structure and hardness of nanocrystalline silver [J]. Mater Sci, 1997, 32(6): 1501-1507.
[20] ZHANG Li-de, MOU Ji-mei. Nanomaterials and nanostructure [M]. 1st ed. Beijing: Science Press, 2002: 216-21. (in Chinese)
[21] QIAN L H, LU Q H, KONG W J, LU K. Electrical resistivity of fully-relaxed grain boundaries in nanocrystalline Cu [J]. Scripta Mater, 2004, 50(11): 1407-1411.
(Edited by LI Xiang-qun)
Foundation item: Project(10475069) supported by the National Natural Science Foundation of China
Corresponding author: LIU Wei; Tel: +86-731-8836791; E-mail: liuweidavid@yahoo.com.cn