J. Cent. South Univ. (2017) 24: 2209-2214
DOI: https://doi.org/10.1007/s11771-017-3629-z
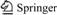
Fuel combustion synthesis and upconversion properties of Yb3+ and Er3+ dual-doped ZrO2 nanocrystals
LIU Xiao-lin(刘小林)1, ZHANG Ning(张宁)2, LI Dan(李丹)1, LI Zhi-cheng(李志成)2,
YOU Wei-xiong(游维雄)1, ZHANG Qian(张骞)1, XIA Li-bin(夏李斌)1, YANG Bin(杨斌)1
1. School of Materials Science and Engineering, Jiangxi University of Science and Technology,Ganzhou 341000, China;
2. School of Materials Science and Engineering, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany 2017
Central South University Press and Springer-Verlag GmbH Germany 2017
Abstract: Ytterbia and erbia dual-doped zirconia (ZrO2: Yb3+, Er3+) nanophosphors were successfully synthesized by high-temperature fuel combustion at 1000 °C for 2 h. The effects of dopant concentration on the structure and upconversion properties were investigated by X-ray diffraction, transmission electron microscopy and photoluminescence, respectively. XRD patterns indicate that the main phase of products belongs to cubic ZrO2 fluorite-type structure. TEM results show that different fuels have great influence on the morphologies of dual-doped ZrO2 samples. Under 980 nm excitation, the glycine-calcined nanophosphors show high stimulated luminescence and doped-ion concentration-depended intensities. The intensely red upconversion emissions are attributed to the fact that the dual-doped Yb3+ and Er3+ ions result in the non-radiative relaxation, energy migration, and cross relaxation.
Key words: nanostructure; luminescence; upconversion; zirconia
1 Introduction
In recent years, rare-earth (RE) compounds and RE ions doped compounds have shown remarkable superiority in field of modern solid state lighting, such as light emitting diodes (LED), fluorescent lamps, field emission displays, and plasma display panels [1–4]. Especially, the use of up-converting RE ions doped nanophosphors has been carried out and sparked considerable interest. For example, intensive attention has been given to Er3+ ion doped nanophosphors emitting in the visible range by the upconversion process, because its metastable levels 4I11/2 can be conveniently populated by near infrared (NIR) excitation (wavelength of 980 nm) [5–10]. In addition, green and red upconversion emissions can be simultaneously realized in Er3+ doped materials due to multi energy level structure of Er3+ ion. In order to improve the pumping efficiency of upconversion in lighting system, another ion is usually introduced into the host materials as a sensitizer. In Er3+ doped materials, the Yb3+ ion is an ideal candidate for sensitizer because it has a broad absorption band around the pump wavelength around 980 nm and a much higher absorption cross-section than the 4I11/2 excited state of Er3+ [11, 12]. In the Yb3+ and Er3+ co-doped systems, the pumping energy can be absorbed by Yb3+ efficiently and transferred to the Er3+ ion to enhance the luminous intensity. Furthermore, the Yb3+ ion has only two energy levels, which excludes its other excited state absorption or upconversion losses in principle [13, 14]. Red and green emission on Er3+ doped and Yb3+—Er3+ dual-doped oxide nanocrystals has been widely reported, such as on gadolinium gallium garnet (GGG), yttrium aluminum garnet (YAG), Lu2O3, ZnO and Y2O3 [15–18], and some works informed strong emission on ZrO2 [19–22]. However, the developed nanocrystalline upconverting phosphors still suffer from the chemical stability or need rigorous synthesis conditions. Therefore, it is very necessary to develop the low-cost and environmental-friendly oxide nanophosphors to meet with the requirements for upconversion photoluminescence.
Ytterbia and erbia dual-doped ZrO2-matrix phosphor is an excellent candidate for use in upconversion luminescence due to its low phonon energy and stable chemical properties. The stretching frequency of the Zr—O bond is about 470 cm–1, which is much lower than that of Al—O (870 cm–1) or Si—O (1100 cm–1) but higher than that of Y—O (300–380 cm–1) [20, 21]. The low phonon energy of such nanocrystal opens up the possibility of higher efficient luminescence of active ions incorporate into the ZrO2 host. Normally, ZrO2 has three kinds of stable structural isomers: the monoclinic phase below 1100 °C, the intermediate tetragonal phase between 1100 and 2370 °C, and the cubic phase above 2370 °C. However, the Yb3+ and Er3+ doped ZrO2 phosphor crystallizes in a rather tetragonal or cubic system than a monoclinic one. Oxygen vacancies, the cluster of earth ion or other impurities in ZrO2: Yb3+, Er3+ systems are easily generated due to charge difference and doped-ion content change, which may deteriorate the luminescence intensity due to non-radiation transition. Therefore, it is desirable to study the role of Yb3+ and Er3+ concentration on the tunability of green-red upconversion emission.
Herein, we report the preparation of ZrO2: Yb3+, Er3+ nanoparticle phosphors with different Yb3+ and Er3+ concentration by combustion synthesis using different fuels. The upconversion luminescence properties of ZrO2: Yb3+, Er3+ phosphors were studied in detail by changing doping concentration of Yb3+ and Er3+ ions in the host. It is found that the ZrO2: Yb3+, Er3+ nanocrystals with required controllable upconverting emission can be obtained at 1000 oC for a short sintering period of 2 h.
2 Experimental
A typical experimental procedure for the ZrO2: 4% Er, 4% Yb sample synthesis as an example is described as follows: 2.459 g ZrO(NO3)2·2H2O, 0.185 g Er(NO3)3·6H2O and 0.180 g Yb(NO3)3·5H2O were successively dissolved in 120 mL deionized water under magnetic stirring at 80 °C, and the solution was continuously stirred until a clear solution was obtained. The co-precipitation reaction immediately started when 1 mol/L ammonia solution was slowly added to the above mixed solution. Meanwhile, the pH value of the mixed solution was adjusted to 10 (pH=10) with slow dropwise of ammonia solution under magnetic stirring at 80 °C. The resultant solution was kept under stirring for about 30 min at 80 °C, and a white precursor product formed. After cooling to room temperature, the white precursor was transferred into 200 mL Teflon-lined autoclave which was sealed and aged at 100 °C for 12 h, after which, the precursor product was separated from the solution by centrifugation and washed with absolute ethanol once and dried at 60 °C. Then, the dried precursor was fully grounded with 1 g of one fuel from glycine, semicarbazlde hydrochloride (SH) or urea, and followed by calcination at 1000 °C for 2 h in air atmosphere to yield the Yb3+ and Er3+ dual-doped ZrO2 powders. Other samples with different Yb3+ and Er3+ concentration were also synthesized by the same procedure with the corresponding starting materials.
X-ray powder diffraction (XRD) patterns of the as-prepared samples were recorded using a Bruker D8-Focus X-ray diffractometer with high-intensity Cu Kα radiation (λ=1.54178 ). Transmission electron microscopy (TEM) micrographs were taken with a Tecnai F20 field-emission transmission electron microscope. Upconversion luminescence and the fluorescence decays at the wavelength of 679 nm were recorded on an Edinburgh instruments FL920 spectrophotometer equipped with laser diode (LD) as the pump source and the excitation wavelength was 980 nm. The signal was detected with a NIR photomultiplier tube (PMT) (R5509, Hamamatsu) and all the spectra have been corrected according to the response curve of the instrument due to the different responses of the instrument at different wavelengths.
). Transmission electron microscopy (TEM) micrographs were taken with a Tecnai F20 field-emission transmission electron microscope. Upconversion luminescence and the fluorescence decays at the wavelength of 679 nm were recorded on an Edinburgh instruments FL920 spectrophotometer equipped with laser diode (LD) as the pump source and the excitation wavelength was 980 nm. The signal was detected with a NIR photomultiplier tube (PMT) (R5509, Hamamatsu) and all the spectra have been corrected according to the response curve of the instrument due to the different responses of the instrument at different wavelengths.
3 Results and discussion
Figure 1 shows the XRD patterns of ZrO2: 4% Er, 4% Yb samples under calcination with different fuels (glycine, SH or urea). In the case of 4% Er and 4% Yb dual-doped ZrO2, the XRD patterns only exhibit characteristic diffraction peaks of single cubic phase (JCPDS No. 49–1642). No diffraction peaks from impurities were observed in those XRD patterns, indicating that the main-phase cubic ZrO2 can be obtained by the fuel combustion method at 1000 °C for 2 h using Zr(OH)4 as precursor in the presence of different fuels. For different doped ion concentration, the XRD patterns for characteristic samples are compared in Figs. 2 and 3. It can be seen that a mixture of tetragonal and cubic phase is observed when the Er3+ concentration is lower than 3%. Notice that dominant peaks in both tetragonal and cubic are coincident and located at 30.12°. Tetragonal phase can be identified by the characteristic double peak (2,0,0)t and (0,1,1)t at 34.92° and 35.40°, and single peak (2,0,0)c at 35.13° for cubic crystalline structure [19]. From XRD patterns of Fig. 2, these results suggest that Er3+ ion concentration has important influence on the stability of crystalline phase. Comparatively, different Yb3+ ion and constant Er3+ concentration (4%) were introduced into host to investigate the phase evolution of ZrO2. Figure 3 shows that the increment of Yb3+ ion concentration and keeping Er3+ concentration consistent (4%) has no significant influence on the crystalline phase composition and peak intensities. All these results also confirm that the dual-addition of more than 3% Er and 1% Yb (molar fraction) to ZrO2 can stabilize the cubic phase.

Fig. 1 XRD patterns of ZrO2: 4% Yb3+, 4% Er3+ prepared by combustion synthesis:
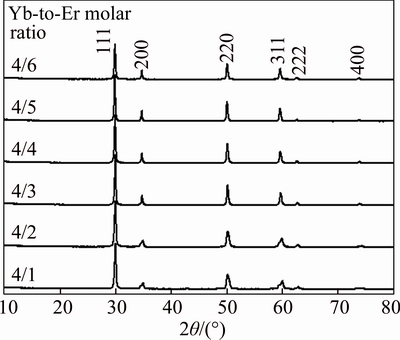
Fig. 2 XRD patterns of dual-doped ZrO2 for 4% Yb3+ and different concentrations of Er3+

Fig. 3 XRD patterns of dual-doped ZrO2 for 4% Er3+ and different concentration of Yb3+
Figure 4 shows the TEM images of ZrO2: 4% Yb3+, 4% Er3+ prepared by the combustion method at 1000 oC for 2 h in the absence or presence of urea, SH and glycine, respectively. One can see that different morphologies of dual-doped ZrO2 were obtained under high-temperature calcination with different fuels (from Figs. 4(a)–(d)), indicating that the types of fuel have great influence on the morphologies of dual-doped ZrO2 samples. In addition, uniform nanoparticles with size of 50 nm are the primary product of the combustion synthesis at 1000 °C when the chosen fuel is glycine (Fig. 4(d)). However, dual-doped ZrO2 samples synthesized by decomposition reaction with the help of other fuels (urea, SH) have large particle size or agglomerate together seriously (>1 μm), which is unfavorable for improving luminescent efficiency [23].
The excitation process for the upconversion emission of Er3+ and Yb3+ ions under excitation wavelength of 980 nm can be illustrated in Fig. 5. The Yb3+ ion can be excited to the sole exited F5/2 level by absorbing the first photon of NIR and then transferred the energy to Er3+ ion. The Er3+ ion is first promoted to the 4I11/2 level, and further to 4F7/2 due to absorption and energy transfer of another NIR photon. Direct excitation is also available, but the energy transfer process is dominant because of the long radiative life time of the excited 2F5/2 level (typically 1 ms) and resonance between 2F5/2→2F7/2 transition of Yb3+ and 4I15/2→4I11/2 transition of Er3+. After being excited to 4F7/2, Er3+ decays rapidly and non-radiatively to the 2H11/2, 4S3/2, 4F9/2 or 4I13/2 levels. As a result, the red emission located at 630–710 nm region is surely assigned to 4F9/2→4I15/2 transition of Er3+ ion. And the green emission located at 510–570 nm region corresponds to 2H11/2→4I15/2 and 4S3/2→4I15/2 transitions of Er3+ ion. Figure 6 shows the upconversion photoluminescence spectra of the 4% Yb3+ and 4% Er3+ dual-doped ZrO2 samples sintered by different fuels under excitation wavelength 980 nm. One can see that the 4% Yb3+, 4% Er3+ dual-doped ZrO2 exhibits very high intensity of the red luminescence and low intensity of the green luminescence (Ired/Igreen>10), perceptible to the naked eyes as a red emission. This quenching of the green luminescence may be due to the cross-relaxation process between Yb3+ ion and Er3+ ion, 2H11/2/4S3/2(Er)+2F7/2(Yb)→4I13/2(Er)+2F5/2(Yb) [12]. In Fig. 6, the highest luminescence intensity is obtained from the 4% Yb3+, 4% Er3+ dual-doped ZrO2 synthesized with glycine used as fuels. This result may be attributed to the small particle size of ZrO2 nanopowders (about 50 nm) by using glycine, whereas the ZrO2 materials prepared with other fuels have large particle size or agglomerate heavily.
The emission spectra as a function of doped-ion concentration are shown in Figs. 7 and 8. As observed in both figures, the upconversion emission is obviously influenced by the concentration of both Yb3+ and Er3+ ions. In Fig. 7, the increment of Er3+ with constant 4% Yb3+ firstly enhances the red band but always quenches the green band. This behavior may be due to the increase in the non-radiative relaxation of 4I11/2→4I13/2 with the Er3+ concentration increment. The non-radiative relaxation Er3+ (4I11/2→4I13/2) increases the population at the 4I13/2 lever, which on time allows an increasing population at the 4F9/2 level via the direct Yb3+ to Er3+ direct energy transfer process Yb3+ (2F5/2, 2F7/2)→Er3+(4I13/2, 4F9/2). Then the emission plots are followed by quenching the red band when the concentration of Er3+ ion is more than 3%. This quenching result may be the association with energy migration, which is caused by the high concentration of doped rare-earth ions. In addition, the increasing Yb3+ has relatively small influence on both bands when the Yb3+ ion increases to a critical concentration of 2% (shown in Fig. 8). Such phenomena shows that 2% Yb3+ in ZrO2 host can provide enough excitation photon for the energy transfer from Yb3+ (donor) to Er3+ (acceptor).

Fig. 4 TEM images of ZrO2: 4% Yb3+, 4% Er3+ prepared by combustion synthesis:

Fig. 5 Schematic for process of Yb3+ sensitized Er3+ upconversion luminescence
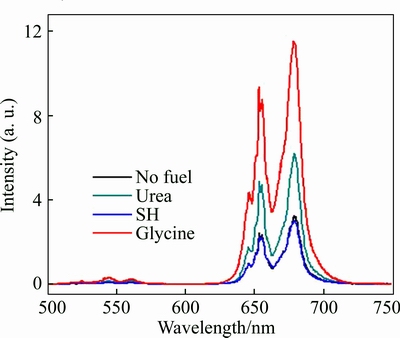
Fig. 6 Upconversion emission spectra of ZrO2: 4% Yb3+, 4% Er3+ for different fuels
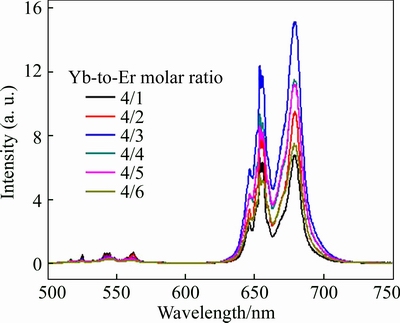
Fig. 7 Upconversion emission spectra of dual-doped ZrO2 prepared by glycine combustion for 4% Yb3+ and different concentrations of Er3+
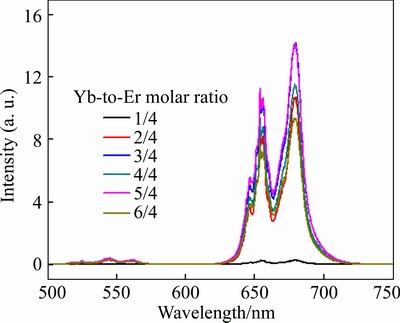
Fig. 8 Upconversion emission spectra of dual-doped ZrO2 prepared by glycine combustion for 4% Er3+ and different concentrations of Yb3+
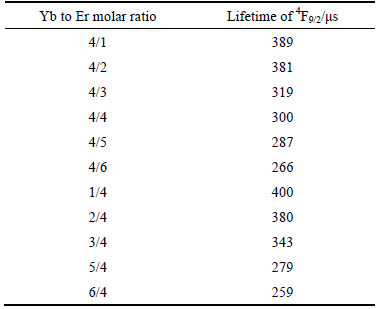
To investigate the influence of ions concentration in the dynamics of upconversion process, Fig. 9 shows the decay curves of Yb3+ and Er3+ dual-doped ZrO2 for red (679 nm) emission. Notice that the fitting of the experiment date is a double exponential function and composed of the first linear and followed slow decay components, which are probably attributed to the cross relaxation described above, non-radiative processes, promotion to higher level, or the multisite nature of the ZrO2 lattice. The decay curves also show that there is significant difference in the fluorescence decay rate when the different Yb3+ or Er3+ ion content is introduced into ZrO2 nanoparticle host. The effective fluorescence lifetime is listed in Table 1 and calculated according to the equation 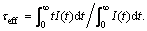 . It can be seen that the decay time decreases with the increase of total doped ion concentration. Such diminish behavior is related to the presence of fluorescence quenching produced by the cluster formation of Er3+ ion due to the increment of concentration which is also responsible of the increase of the upconversion signal intensity.
. It can be seen that the decay time decreases with the increase of total doped ion concentration. Such diminish behavior is related to the presence of fluorescence quenching produced by the cluster formation of Er3+ ion due to the increment of concentration which is also responsible of the increase of the upconversion signal intensity.
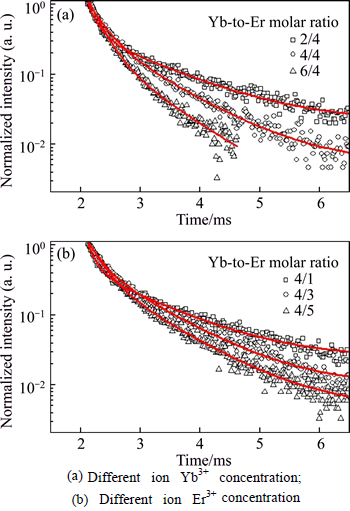
Fig. 9 Typical decay time curves of dual-doped ZrO2:
Table 1 Lifetime of 4F9/2 level in different Yb3+ and Er3+ concentration doped samples
4 Conclusions
Yb3+ and Er3+ co-doped ZrO2 phosphors have been successfully synthesized by the combustion synthesis at 1000 °C using ZrO(NO3)2·2H2O, Er(NO3)3·6H2O and Yb(NO3)3·5H2O as starting raw materials. And the nanosized Yb3+ and Er3+ co-doped ZrO2 particles can be easily and successfully manipulated using different fuels. Luminescence results show that the as-formed Yb3+ and Er3+ dual-doped ZrO2 nanoparticles exhibit highly and controllable luminescent intensity by adjusting the appropriate concentration of doped Yb3+ and Er3+ ions. Cross-relaxation processes may be the main reason of weak green and strong red luminescence at the same time. Moreover, the luminescence decay characteristics of lifetime decrease with the increment of total doped ion concentration. Those results show that in Yb3+ and Er3+ dual-coped ZrO2 system, doping ion concentration plays an important role in the high-intensity red upconversion emissions.
References
[1] ZHOU Jun, XIA Zhi-guo. Luminescence properties and energy transfer studies of a color tunable BaY2Si3O: Tm3+, Dy3+ phosphor [J]. Optical Materials, 2016, 53: 116–122.
[2] CHEN Ai-min, WANG Jing, BO Ying-ying, LIU Rui, GU Pei, PAN Zai-fa. Synthesis, characterization and photoluminescence properties of hierarchical Mg3B2O6: Eu3+ flower-like microspheres [J]. Chinese Journal of Inorganic Chemistry, 2015, 31: 1548–1554.
[3] WU Su-li, LIU Ye, CHANG Jie, NING Yan-hui, ZHANG Shu-fen. Beta-NaYF4: Yb3+, Er3+ upconversion microcrystals with both high emission intensity and controlled morphology [J]. Laser & Photonics Reviews, 2014, 8: 575–582.
[4] GU Ying-ying, LI Lu-ke, ZHANG Wen-wen, LIU Ying, LU Zhou-guang. Precipitation processes and luminescence properties of ZnO: La3+, Li+ nanoparticles [J]. Journal of Central South University, 2013, 20(2): 332–336.
[5] AISAKA T, FUJII M, HAYASHI S. Enhancement of upconversion luminescence of Er doped Al2O3 films by Ag island films [J]. Applied Physics Letters, 2008, 92: Art. No. 132105.
[6] VETRONE F, BOYER J C, CAPOBIANCO J A, SPEGHINI A, BETTINELLI M. Effect of Yb3+ codoping on the upconversion emission in nanocrystalline Y2O3:Er3+ [J]. Journal of Physical Chemistry B, 2003, 107: 1107–1112.
[7] VETRONE F, NACCACHE R, de La FUENTE A J, SANZ-RODRIGUEZ F, BLAZQUEZ-CASTRO A, RODRIGUEZ E M, JAQUE D, SOLE J G, CAPOBIANCO J A. Intracellular imaging of HeLa cells by non-functionalized NaYF4:Er3+,Yb3+ upconverting nanoparticles [J]. Nanoscale, 2010, 2: 495–498.
[8] MAHALINGAM V, MANGIARINI F, VETRONE F, VENKATRAMU V, BETTINELLI M, SPEGHINI A, SPEGHINI J A. Bright white upconversion emission from Tm3+/Yb3+/Er3+ doped Lu3Ga5O12 nanocrystals [J]. Journal of Physical Chemistry C, 2008, 112: 17745–17749.
[9] IMANIEH M H, MARTIN I R, NADARAJAH A, LAWRENCE J G, LAVIN V, GONZALEZ-PLATAS J. Upconversion emission of a novel glass ceramic containing Er3+, Yb3+: Sr1–xYxF2, nanocrystals [J]. Journal of Luminescence, 2016, 172: 201–207.
[10] URBINA-FRIAS A, LOPEZ-LUKE T, OLIVA J, SALAS P, TORRES-CASTRO A, DE LA ROSA E. Strong enhancement of the upconversion emission in ZrO2: Yb3+, Er3+, Gd3+ nanocubes synthesized with Na2S [J]. Journal of Luminescence, 2016, 172: 154–160.
[11] YOU Wei-xiong, HUANG Yi-dong, CHEN Yu-jin, LIN Yan-fu, LUO Zun-du. The Yb3+ to Er3+ energy transfer in YAl3(BO3)4 crystal [J]. Optics Communications, 2008, 281: 4936–4939.
[12] YOU Wei-xiong, LAI Feng-qin, JIANG Hong-hui, LIAO Jin-sheng. The up-conversion properties of Yb3+ and Er3+ doped Y4Al2O9 [J]. Physica B—Condensed Matter, 2012, 407: 1094–1098.
[13] CANTELAR E, MUNOZ J A, SANZ-GARICIA J A, CUSSO F. Yb3+ to Er3+ energy transfer in LiNbO3 [J]. Journal of Physics: Condensed Matter, 1998, 10: 8893–8903.
[14] DA VILA L D, GOMES L, TARELHO L V G, RIBEIRO S J L, MESADEQ Y. Mechanism of the Yb-Er energy transfer in fluorozirconate glass [J]. Journal of Applied Physics, 2003, 93: 3873–3880.
[15] KRSMANOVIC R, MOROZOV V A, LEBEDEV O I, POLIZZI S, SPEGHINI A, BETTINELLI M, TENDELOO G V. Structural and luminescence investigation on gadolinium gallium garnet nanocrystalline powders prepared by solution combustion synthesis [J]. Nanotechnology, 2007, 18: Art. No. 325604.
[16] FEI Bin-jin, CHEN Wei-dong, GUO Wang, SHI Ming, LIN Hai-feng, HUANG Qiu-feng, ZHANG Ge, CAO Yong-ge. Optical properties and laser oscillation of Yb3+, Er3+ co-doped Y3Al5O12 transparent ceramics [J]. Journal of Alloys and Compounds, 2015, 636: 171–175.
[17] ZHENG Ke-zhi, SONG Wei-ye, LV Chang-jiang, LIU Zhen-yu, QIN Wei-ping. Controllable synthesis and size-dependent upconversion luminescence properties of Lu2O3: Yb3+/Er3+ nanospheres [J]. CrystEngComm, 2014, 16: 4329–4337.
[18] FENG Wei, WANG Xiang-fu, MENG Lan, YAN Xiao-hong. Fabrication and photoelectric properties of Er3+ and Yb3+ co-doped ZnO films [J]. Japanese Journal of Applied Physics, 2016, 55: Art. No. 012302.
[19] SOLIS D, DE LA ROSA E, MEZA O, DISZ-TORRES L A, SALAS P, ANGELES-CHAVEZ C. Role of Yb3+ and Er3+ concentration on the tunability of green-yellow-red upconversion emission of codped ZrO2:Yb3+-Er3+ nanocrystals [J]. Journal of Applied Physics, 2010, 108: Art. No. 023103.
[20] DING Jia-feng, LI Xin-mei, CUI Li-ling, CAO Can, WANG Hui-hai, CAO Jian. Electronic and optical properties of anion-doped c-ZrO2 from first-principles calculations [J]. Journal of Central South University, 2014, 21(7): 2584–2589.
[21] PATRA A, FRIEND C S, KAPOOR R, PRASAD P N. Upconversion in Er3+: ZrO2 nanocrystals [J]. The Journal of Physical Chemistry B, 2002, 106: 1909–1912.
[22] DIAZ-TORRES L A, MEZA O, SOLIS D, SALAS P, DE LA ROSA E. Visible upconversion emission and non-radiative direct Yb3+ to Er3+ energy transfer processes in nanocrystalline ZrO2:Yb3+, Er3+ [J]. Optics and Lasers in Engineering, 2011, 49: 703–708.
[23] PARK Y I, NAM S H, KIM J H, BAE Y M, YOO B, KIM H M, JEON K S, PARK H S, CHOI J S, LEE K T, SUH Y D, HYEON T. Comparative study of upconverting nanoparticles with various crystal structures, core/shell structures, and surface characteristics [J]. The Journal of Physical Chemistry C, 2013, 117: 2239–2244.
(Edited by FANG Jing-hua)
Cite this article as: LIU Xiao-lin, ZHANG Ning, LI Dan, LI Zhi-cheng, YOU Wei-xiong, ZHANG Qian, XIA Li-bin, YANG Bin. Fuel combustion synthesis and upconversion properties of Yb3+ and Er3+ dual-doped ZrO2 nanocrystals [J]. Journal of Central South University, 2017, 24(10): 2209–2214. DOI:https://doi.org/10.1007/s11771-017-3629-z.
Foundation item: Projects(51062004, 11464017) supported by the National Natural Science Foundation of China; Project(GJJ150671) supported by the Scientific Research Foundation for Universities from Education Bureau of Jiangxi Province, China; Project(20161BAB216123) supported by the Natural Science Foundation of Jiangxi Province, China; Projects(NSFJ2014-G13, Jxxjbs12005) supported by the Jiangxi University of Science and Technology, China
Received date: 2016-04-12; Accepted date: 2016-12-01
Corresponding author: LIU Xiao-lin, Lecturer, PhD; Tel: +86–797–8312078; E-mail: xlliu@jxust.edu.cn