Iron and copper recovery from copper slags through smelting with waste cathode carbon from aluminium electrolysis
来源期刊:中南大学学报(英文版)2021年第7期
论文作者:李磊 毛凯旋 徐苗
文章页码:2010 - 2021
Key words:copper slags; waste cathode carbon; oxidative desulfurization; smelting reduction; iron and copper recovery; fluoride
Abstract: To recover metal from copper slags, a new process involving two steps of oxidative desulfurization followed by smelting reduction was proposed in which one hazardous waste (waste cathode carbon) was used to treat another (copper slags). The waste cathode carbon is used not only as a reducing agent but also as a fluxing agent to decrease slag melting point. Upon holding for 60 min in air atmosphere first and then smelting with 14.4 wt% waste cathode carbon and 25 wt% CaO for 180 min in high purity Ar atmosphere at 1450 °C, the recovery rates of Cu and Fe reach 95.89% and 94.64%, respectively, and meanwhile greater than 90% of the fluoride from waste cathode carbon is transferred into the final slag as CaF2 and Ca2Si2F2O7, which makes the content of soluble F in the slag meet the national emission standard. Besides, the sulphur content in the obtained Fe-Cu alloy is low to 0.03 wt%.
Cite this article as: MAO Kai-xuan, LI Lei, XU Miao. Iron and copper recovery from copper slags through smelting with waste cathode carbon from aluminium electrolysis [J]. Journal of Central South University, 2021, 28(7): 2010-2021. DOI: https://doi.org/10.1007/s11771-021-4749-z.

J. Cent. South Univ. (2021) 28: 2010-2021
DOI: https://doi.org/10.1007/s11771-021-4749-z
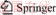
MAO Kai-xuan(毛凯旋)1, 2, 3, LI Lei(李磊)1, 2, 3, XU Miao(徐苗)1, 2, 3
1. State Key Laboratory of Complex Non-ferrous Metal Resources Clean Utilization,
Kunming University of Science and Technology, Kunming 650093, China;
2. Engineering Research Center of Metallurgical Energy Conservation and Emission Reduction of
Ministry of Education, Kunming University of Science and Technology, Kunming 650093, China;
3. Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Abstract: To recover metal from copper slags, a new process involving two steps of oxidative desulfurization followed by smelting reduction was proposed in which one hazardous waste (waste cathode carbon) was used to treat another (copper slags). The waste cathode carbon is used not only as a reducing agent but also as a fluxing agent to decrease slag melting point. Upon holding for 60 min in air atmosphere first and then smelting with 14.4 wt% waste cathode carbon and 25 wt% CaO for 180 min in high purity Ar atmosphere at 1450 °C, the recovery rates of Cu and Fe reach 95.89% and 94.64%, respectively, and meanwhile greater than 90% of the fluoride from waste cathode carbon is transferred into the final slag as CaF2 and Ca2Si2F2O7, which makes the content of soluble F in the slag meet the national emission standard. Besides, the sulphur content in the obtained Fe-Cu alloy is low to 0.03 wt%.
Key words: copper slags; waste cathode carbon; oxidative desulfurization; smelting reduction; iron and copper recovery; fluoride
Cite this article as: MAO Kai-xuan, LI Lei, XU Miao. Iron and copper recovery from copper slags through smelting with waste cathode carbon from aluminium electrolysis [J]. Journal of Central South University, 2021, 28(7): 2010-2021. DOI: https://doi.org/10.1007/s11771-021-4749-z.
1 Introduction
The copper slag is a hazardous waste which is generated from the cleaning process of copper converter slag or smelting slag in an electrical furnace. Generally, 2.0-3.0 t of copper slags are generated per ton of copper produced in the copper pyrometallurgical process, and approximately 30 million tons of slags are deposited every year in the world [1-3]. Most of them are directly discharged into the environment without treatment, causing serious economic and environmental issues [4]. Hence, the utilization of copper slags as different value added products, such as abrasive tools, pavements, glasses, cements, roofing granules, tiles, and asphalt concrete aggregates, has been explored [5, 6]. In addition, the copper slag typically contains approximately 35 wt%-45 wt% Fe and 0.5 wt%-1.5 wt% Cu, which suggests that it is also a valuable resource for metal recovery [7]. Different methods for copper and iron recovery have been researched in recent years. These methods involve hydrometallurgical, pyrometallurgical and physical processes [8, 9]. A high copper recovery rate could be obtained through hydrometallurgical processes, but the waste leaching liquid presents a new form of environmental pollution [10]. Physical processes primarily include flotation to recover the copper and magnetic separation to recover the iron. The flotation process is only suitable for extracting sulphide minerals and has little effect on the recovery of copper oxides. Meanwhile, magnetic separation yields a low iron recovery rate because most iron exists in the form of fayalite (Fe2SiO4) in the copper slag [11]. In comparison, the pyrometallurgical processes have been widely used in previous researches given the advantages that the copper slag can be treated under molten conditions once it is discarded from the furnace before it cools down, thus achieving full use of heat value [12]. High recovery rates of copper and iron are obtained by producing Fe-Cu alloy (iron rich alloy) using a carbothermal or aluminothermy reduction process [4, 13], and this process produces low level of environmental pollution. The pyrometallurgical process is actually a slag making process, and the slag viscosity affects significantly the separation of slag and metal and the metal recovery rates. HEO et al [14] investigated the effect of CaO amount on the Fe recovery rate from copper slags by a carbothermal reduction process, and found that the Fe recovery rate reached over 90% with 20 wt% CaO added at 1500 °C for 60 min due to no formation of solid compounds with high melting point and the slag was therefore highly fluid. GUO et al [15] found that with the addition of 10 wt% Na2CO3, the reduction of the fayalite, copper sulfide and copper silicate could be promoted through a phase transformation, and the metallization rate of the Fe and Cu in the reduced pellets increased from 67.78% to 93.21% and from 69.54% to 83.45%, respectively [15]. However, the S element originated from copper slags enters into the Fe-Cu alloy in the form of Cu2S and/or FeS, reducing the alloy property obtained. This feature has received minimal consideration [16].
In addition, we have noticed that the waste cathode carbon, which is generated from the primary aluminium industry, is mainly composed of carbon, sodium fluoride and other compounds. Considerable amounts of this waste have accumulated, occupying large amounts of land in recent years [17, 18]. The fluoride and cyanide components in it cause environment pollution if exposed to air for a long time. It is regarded as a hazardous waste by various environmental bodies. Many hydrometallurgical or pyrometallurgical processes have been used to treat waste cathode carbon to attain harmlessness and carbon separation [19-21]. Due to the difference in hydrophobic water between carbon and electrolyte, the electrolytes and carbon powder could be obtained and recovered from the waste cathode carbon [22]. However, the toxic HCN and HF would be released and precipitated during the treatment [23], which cause serious equipment corrosion and environment pollution. With the additive of fly ash or limestone, the decomposition rate of cyanide obtained 100% at temperature over 700 °C, and meanwhile most of the fluoride is solidified in the form of CaF2 to meet the national emission standard [23, 24]. The carbon material can not been fully used through the processes above. The flotation technology is not suitable to improve the carbon purity and recovery efficiency of the leachable substance. Although thermal treatment reduces the amount of waste of some refractory components, the carbon source is not reused.
Moreover, paying attention to the fluoride component, which exists mainly in forms of NaF, Na3AlF6 and CaF2 in the waste cathode carbon and can be used as a fluxing agent in the pyrometallurgical process through decreasing the slag melting point, an innovative technology that involves oxidative desulfurization and a smelting reduction process followed by the use of waste cathode carbon was proposed to produce a Fe-Cu alloy containing minimal sulphur from copper slags in this paper. In addition, most fluoride was transferred into the slag phase with low leachability, and the generated Fe-Cu alloy could be further used to produce weathering steel [25, 26].
2 Materials and methods
2.1 Materials
2.1.1 Copper slags
The copper slag in this work was obtained from Yunnan Copper Co., Ltd, China. After crushing and grinding, chemical titrimetry analysis was used to determine its composition, and the result is shown in Table 1. The slag contains 0.73 wt% Cu, 39.88 wt% Fe and 1.14 wt% S. Figure 1(a) shows the X-ray diffraction (XRD) pattern, which identifies fayalite and magnetite as the main phases in the copper slag. The magnetite iron content was detected by a magnetic analyser, and the value is 4.90 wt%, indicating that the iron cannot be recovered effectively through a magnetic separation. In addition, the SEM-EDS analysis result in Figure 2 shows the copper is mainly distributed in the copper slag in the form of sulfide (matte).
Table 1 Chemical composition of copper slag (wt%)

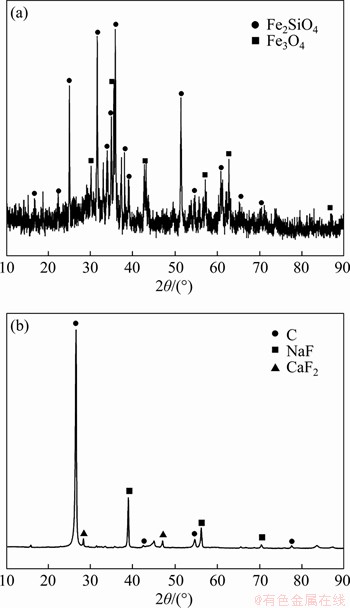
Figure 1 XRD patterns of copper slag (a) and waste cathode carbon (b)
2.1.2 Waste cathode carbon
The waste cathode carbon used, which was the first cut of the spent pot liner, was obtained from Yunnan Aluminum Co., Ltd, China. The results of the proximate analysis of the waste cathode carbon are shown in Table 2 and the chemical analysis of the ash in it is shown in Table 3. It is shown that the waste cathode carbon contains 73.24 wt% fixed carbon, 25.81 wt% ash and 0.85 wt% volatile matters. In addition, 9.49 wt% F, 8.44 wt% Na, 3.59 wt% Al2O3 and a small amount of CN- exist in the ash.
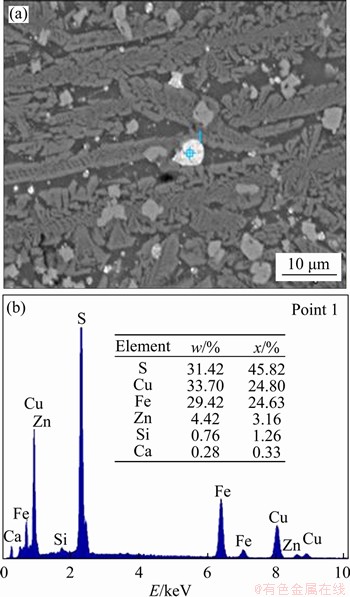
Figure 2 SEM image (a) and EDS analysis (b) of copper phase in raw copper slag
Table 2 Proximate analysis result of waste cathode carbon (wt%)

Table 3 Chemical composition of ash in waste cathode carbon (wt%)

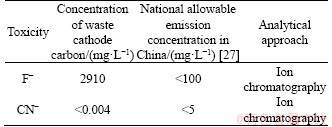
Figure 1(b) identifies C, NaF and CaF2 as main phases in the waste cathode carbon. This waste cathode carbon is listed as a hazardous material given its levels of leachable F- in Table 4. Besides, the CN leaching toxicity in this waste cathode carbon is far less than the national allowable emission concentration in China as shown in Table 4, and it was not researched in this work.
2.1.3 Additive and gas
In this study, the analytical grade CaO was used as an additive. In addition, the purity of Ar gas used in this study was higher than 99.99 vol%.
Table 4 Detection of leaching toxicity of waste cathode carbon
2.2 Methods
The copper slag mainly consists of various oxides and silicates as well as sulphides. Reduction of oxides and silicates leads to recovery of various metals; however, the sulphur element simultaneously enters into the metal phase based on previous research [16], reducing the metal quality obtained. Two steps of treatment of copper slags, including oxidative desulfurization and smelting reduction, were performed in this study.
For the experimental procedure, 30 g cold copper slags with the particle size less than 149 μm were firstly placed in a corundum crucible and then located in a vertical resistance furnace for melting and oxidative desulfurization under air atmosphere. After holding for 60 min at a proper temperature, a certain amount of the mixture of CaO and waste cathode carbon with the particle size less than 149 μm was added into the corundum crucible through a feeder under high purity argon atmosphere. The CaO or waste cathode carbon amount used is expressed as the mass ratio of CaO or waste cathode carbon to copper slags. After 180 min, the sample was then cooled down to room temperature in high purity Ar gas. Once the cooling stage was completed, the sample was removed from the furnace and prepared for analysis.
2.3 Characterization
The chemical composition of the sample was detected by chemical titrimetry, and the average of three measurements was taken as the result. The magnetite iron content in the raw copper slag was measured using a saturated magnetic analyser (SATMAGAN 135), which was carried out in the conditions of voltage of 220 V, frequency of 50 Hz, and testing temperature of 25 °C. The phase composition of the sample was identified by X-ray diffraction (XRD, Rigaku, TTR-III). The diffraction was measured using Cu Kα radiation at 40 kV and 40 mA and a step size of 0.01°. A scanning electron microscope (SEM; HITACHI-S3400 N) coupled with energy dispersive X-ray spectroscopy (EDS) and electron probe microanalysis techniques (EPMA, JXA82, JEOL) were used to determine the phase transformation of the sample in the smelting reduction process. The leachability of the F- and CN- in the sample was measured by Chinese standard leaching test according to the GB5085.3-2007 method. The thermodynamic data of species were given by FactSage 7.2 thermochemical software. During the smelting reduction process, the recovery rates of iron (RFe) and copper (RCu) were defined as follows:
 (1)
(1)
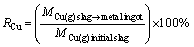 (2)
(2)
where MFe(g)slag→metal ingot and MCu(g) slag→metal ingot are the mass of iron and copper transferred into the metal ingot, and MFe(g)initial slag and MCu(g)initial slag are mass of iron and copper in the raw copper slags, respectively. In addition, the volatilization rate of fluoride (VF) was defined as follows:
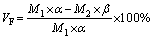 (3)
(3)
where M1 and M2 represents the mass of the waste cathode carbon and final slag, respectively; α and β represent the mass fraction of fluorine in the waste cathode carbon and final slag, respectively.
3 Theoretical analysis of metal recovery
The sulphur mostly occurs in Cu2S and FeS in the copper slag and its content reaches 1.14 wt% as shown in Table 1, most of which enters into the metal phase in the smelting process causing the metal property to be decreased. An oxidative desulfurization process was first performed, in which the sulphur was removed in the form of SO2(g) as [16]:
FeS(l)+1.5O2(g)+xSiO2(g)=FeO·xSiO2(l)+SO2(g) (4)
Cu2S(l)+1.5O2(g)+ySiO2(g)=Cu2O·ySiO2(l)+SO2(g) (5)
Then, with the addition of waste cathode carbon, the reduction of iron and copper compounds in the molten copper slag occurs as follows:
C(s)+CO2(g)=2CO(g) (6)
C(s)+Cu2SiO3(l)=2Cu(l)+CO(g)+SiO2(s) (7)
CO(g)+Cu2SiO3(l)=2Cu(l)+CO2(g)+SiO2(s) (8)
4C(s)+2Fe3O4(s)+3SiO2(s)=3Fe2SiO4(l)+4CO(g) (9)
2CO(g)+2Fe3O4(s)+3SiO2(s)=3Fe2SiO4(l)+2CO2(g) (10)
2C(s)+Fe2SiO4(l)=2CO(g)+2Fe(l)+SiO2(s) (11)
2CO(g)+Fe2SiO4(l)=2CO2(g)+2Fe(l)+SiO2(s) (12)
During the reduction process, Figure 3(a) shows that the fluoride originated from waste cathode carbon almost volatilizes into the gas phase in forms of SiF4 (g), SiF3 (g) and NaAlF4 (g) without CaO addition, which represents environmental pollution risks. Then with the addition of 10 wt%-25 wt% CaO, most fluoride is transformed into calcium fluoride (CaF2(s)) and/or cuspidine (Ca4Si2F2O7 (s)) through reactions (13) and (14) retaining in the slag phase as shown in Figures 3(b)-(d), through which the level of leachable F- might be decreased. Figures 3(b)-(d) also suggest that increasing the amount of CaO from 10 wt% to 20 wt% and 25 wt%, respectively, the CaF2 (s) is almost converted to Ca4Si2F2O7 (s) through reaction (14), and meanwhile the fluoride amount retaining in the slag increases obviously. In addition, the increasing temperature decreases the formation amounts of CaF2 (s) and Ca4Si2F2O7 (s) due to the increased formation of the gas phase of fluoride.
2NaF(l)+CaO(s)+SiO2(s)=CaF2(s)+Na2SiO3(l) (13)
3CaO(s)+2SiO2(s)+CaF2(s)=Ca4Si2F2O7(s) (14)
4 Results and discussion
4.1 Oxidative desulfurization
The raw copper slag was heated at 10 K/min to the target temperature and then held for 60 min in air atmosphere to achieve desulfurization via Eqs. (4) and (5). Figure 4(a) shows that the sulphur residual content decreases obviously as the temperature ranges from 1400 to 1450 °C, and then there is no significant change as the temperature continues to increase. Meanwhile, part of the fayalite (Fe2SiO4) phase is oxidized to Fe3O4 as presented in Figure 4(b). After oxidative desulfurization treatment, the diffractions due to fayalite taper off, whereas those due to magnetite are gradually enhanced.
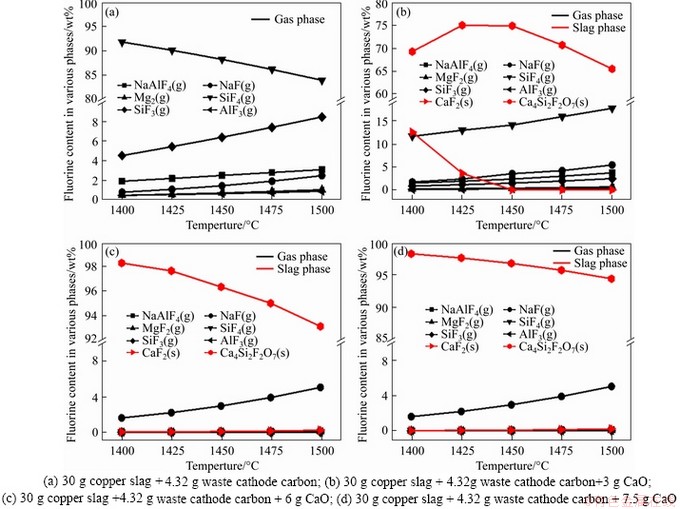
Figure 3 Effects of temperature and CaO amount on equilibrium composition of fluoride phase:
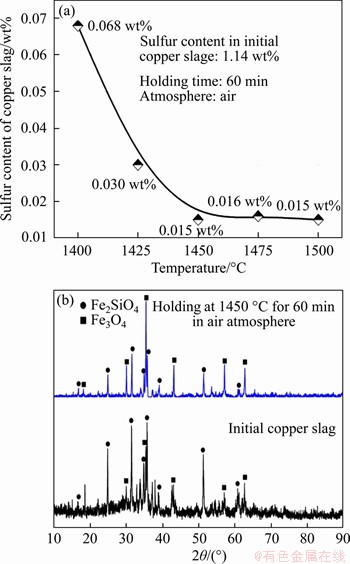
Figure 4 Sulphur content of slag after melting at different temperatures for 60 min in air atmosphere (a) and XRD pattern of raw slag and slag after oxidative desulfurization treatment (b)
4.2 Metal recovery
After the copper slag treated by the process of oxidative desulfurization, the waste cathode carbon was added into the molten slag for reduction and recovery of copper and iron. The parameters of waste cathode carbon mass, calcium oxide addition mass and smelting temperature were studied.
4.2.1 Effect of waste cathode carbon amount
The effect of waste cathode carbon on the smelting reduction process was studied without adding CaO. As the waste cathode carbon amount increases from 12 wt% to 14.4 wt%, more iron and copper could be reduced through reactions (7)-(12) and then transferred into the metal ingot. Meanwhile, the mass of CaF2 and NaF that originated from the waste cathode carbon introduced into the slag are also increased, causing the slag melting point to be obviously decreased as noted in Table 5 and then the metal settling separation and recovery to be further promoted. Consequently, with smelting at 1450 °C for 180 min, the iron recovery rate (RFe) increases from 54.41% to 73.91% with the increase of waste cathode carbon amount from 12 wt% to 14.4 wt% (Table 5) and then increases slightly. In addition, the changes of RCu with the waste cathode carbon amount are similar to that of RFe seen from Table 5. At the same amount of fixed carbon, it is noteworthy that the recovery rates of iron and copper with the addition of waste cathode carbon are obviously higher than that with the coke added as shown in Figure 5, which might be related to the effects of “F” component from the waste cathode carbon. Based on the solubility change of C in the Fe-Cu-C system as a function of Cu content presented in Figure 6 [28], the elements of Cu, C, and Fe exist in the state of mutual soluble in the metal ingot.
Regarding the waste cathode carbon melted with copper slags for 180 min, Table 5 and Figure 7 show that most fluoride is retained in the slag in the form of CaF2. Figure 7 shows the EPMA result of the final slag with the addition of 14.4 wt% waste cathode carbon, in which most F coexists closely with Ca and exists mainly in the form of CaF2 deduced from the composition of “1” point in Figure 7. The level of leachable F- in the final slag might be decreased due to this transformation, which will be confirmed in the subsequent detections. Meanwhile, it is noteworthy that the fluoride volatilization rate (VF) decreases as the amount of waste cathode carbon increases (Table 5), which might be attributed to the fact that more SiO2 could be released from the fayalite (Fe2SiO4) through Eqs. (11) and (12) and then the CaF2 formation through Eq. (13) is promoted. In addition, all the sulphur content in the metal ingot is less than 0.1 wt% (Table 5), which is decreased by a large amount compared to that in the direct smelting reduction of copper slags as reported by previous researches [16]. Regarding the iron and copper recovery rates, the waste cathode carbon amount was fixed at 14.4 wt%.
Table 5 Effect of mass of waste cathode carbon amount, mass of calcium oxide addition and smelting temperature on smelting reduction of copper slags

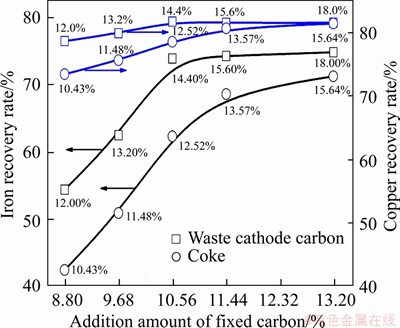
Figure 5 Changes of iron and copper recovery rates with addition of coke and waste cathode carbon respectively
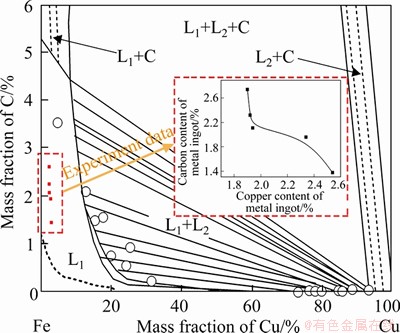
Figure 6 Solubility of C in Fe-Cu-C system as a function of Cu content at 1450 °C
4.2.2 Effect of calcium oxide addition amount
In the smelting process, the slag melting point affects the obtained metal droplet settlement, aggregation, growth and subsequent metal recovery rate. Generally, a low slag melting point promotes the separation between phases of slag and metal. The phase diagram of the SiO2-CaO-Al2O3 system at 1450 °C (Figure 8(a)) determined by Factsage.7.2 software shows that the composition of the slag after the Fe completely reduction without CaO added “A” lies in the region of slag-liq + SiO2(s). The appearance of SiO2 (s) indicates that the molten slag is a solid-containing melt due to a high melting point of SiO2 (1723 °C), which causes the slag viscosity to be increased and the separation of slag and metal to be restricted. Thus, some metal particles might be dispersed in the slag as shown in Figure 9 when the smelting reduction process carried out at 1450 °C for 180 min with the addition of 14.4% waste cathode carbon was finished. Figure 9 shows that some particles of Fe (point 1) and Cu (point 2) are lost in the slag. Correspondingly, the recovery rates of Fe and Cu are low (Table 5). The addition of 10 wt%- 25 wt% calcium oxide changes the slag composition along the indicated red arrow from the slag-liq + SiO2(s) region to the slag-liq region in Figure 8(a). Though the slag melting point increases with the CaO amount from 10 wt% to 25 wt% as shown in Table 5, the complicated silicate structure in the slag could be simplified due to the addition of CaO [29]. As a result, the slag surface tension increases (Figure 8(b)), which promotes the separation between phases of obtained metal and slag. Consequently, the recovery rates of Fe and Cu are improved obviously (Table 5). The surface tension data in Figure 8(b) was calculated according to ion and molecule coexistence theory of slag structure and Bulter’s equation [30-32]. However, further increasing the calcium oxide amount to 30 wt%, both the recovery rates of copper and iron decrease slightly, which might be due to the generation of Ca2Al2SiO7 (Figure 10(a)) of a high melting point and the subsequent increase of slag melting point (Table 5).
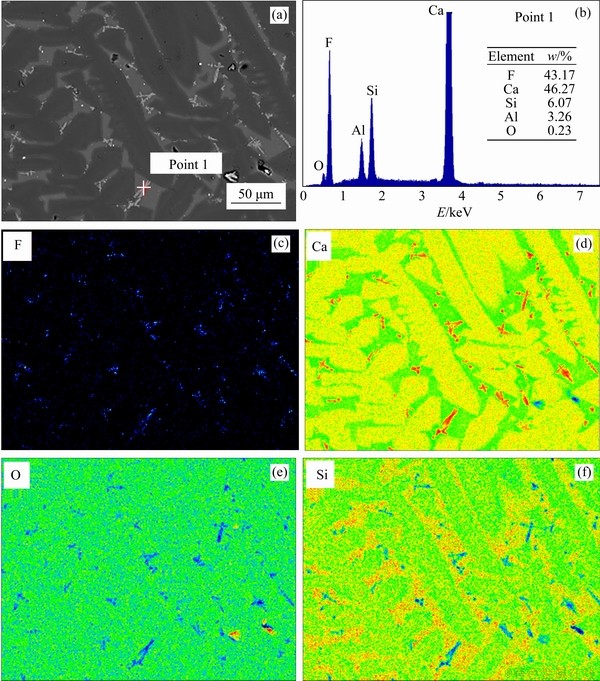
Figure 7 EPMA image of final slag with 14.4 wt% waste cathode carbon addition at 1450 °C (a) and EDS analysis of Point 1 (b-f)
As more CaO is added, more sulphur will be transferred from the metal ingot to the slag phase according to Eq. (15), and correspondingly the sulphur content in the metal ingot decreases as shown in Table 5. In addition, more fluoride will also be transferred into the slag phase in the form of CaF2 (Figure 10(a)) and Ca4Si2F2O7 (Point 1 in Figure 10(b)) by reactions of (13) and (14), and VF decreases (Table 5). Increasing recovery rates of Cu and Fe and decreasing fluoride volatilization, the calcium oxide amount should be controlled at 25 wt%.
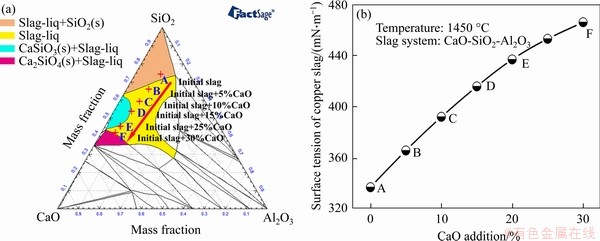
Figure 8 Phase diagram of SiO2-CaO-Al2O3 system at 1450 °C (a) and effect of CaO amount on SiO2-CaO-Al2O3 molten slag surface tension (b)
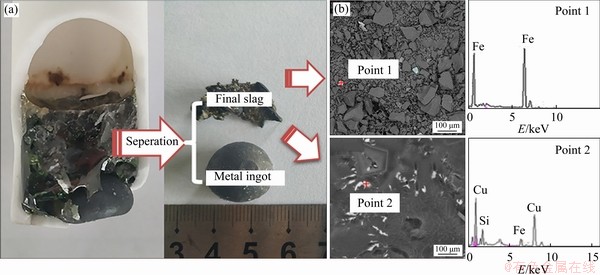
Figure 9 Photo (a) and analysis SEM-EDS (b) of final slag smelted at 1450 °C with no CaO added
(CaO)+[S]=(CaS)+[O] (15)
4.2.3 Effect of smelting temperature
The effects of smelting temperature were assessed in the range of 1400-1500 °C under the condition of waste cathode carbon amount of 14.4 wt%, calcium oxide amount of 25% and smelting time of 180 min. The results are shown in Table 5. The RCu changes slightly as the temperature increases from 1400 to 1500 °C. The RFe first increases from 93.34% to 94.64% as the temperature increases from 1400 to 1450 °C and then remains almost constant around a value of 94% as the temperature continues to increase. In addition, it is noteworthy that the increase in smelting temperature improves the volatilization rate of fluoride from Table 5, which is consistent with the calculation results in Figure 3. The temperature ranging from 1400 to 1450 °C might be a suitable smelting temperature.
After the copper slag is first treated in air atmosphere for desulfurization and then smelting reduced using 14.4% waste cathode carbon for metal recovery, a metal ingot with minimal S content (0.03 wt%) is obtained. Meanwhile, greater than 90% of the fluoride originated from the waste cathode carbon is transferred into the slag with 25 wt% CaO added at 1450 °C. Moreover, the value of leachable F- in the final slag was detected via ion chromatography method and found to be 3.11 mg/L,which is far less than the national allowable emission concentration in China as can be seen in Table 6.
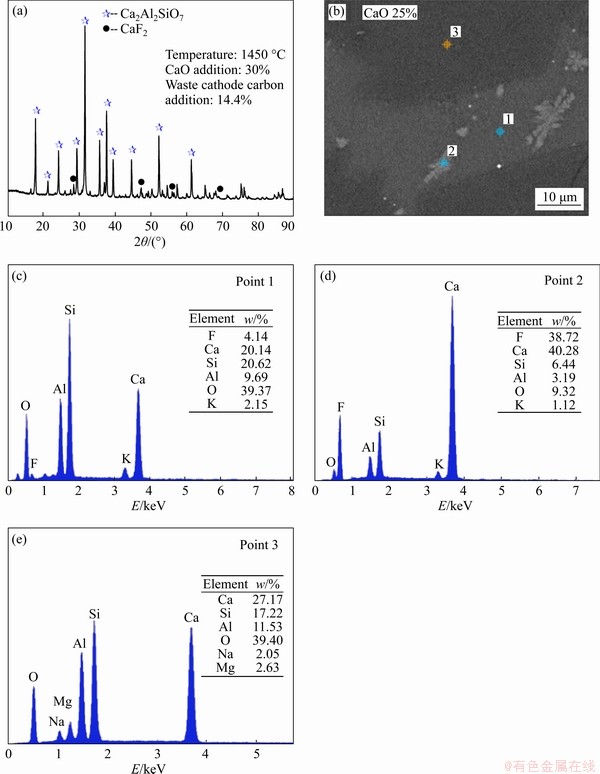
Figure 10 XRD pattern of final slag with 30 wt% CaO addition at 1450 °C (a), EPMA image of final slag with 25 wt% CaO addition at 1450 °C (b), and corresponding EDS analyses of Points 1-3 (c-e)
Table 6 Detection of leaching toxicity of final slag

5 Conclusions
1) A high recovery rate of iron and copper and a significant decrease in the level of leachable F- can be obtained when copper slags are smelted with waste cathode carbon.
2) The waste cathode carbon is listed as a hazardous material given its high level of leachable F-. When smelted with copper slags, most of this fluoride is transferred into the final slag in forms of CaF2 and Ca4Si2F2O7, and the increase in the addition of CaO promotes this transformation. Thus, the level of leachable F- decreases significantly and is far less than the national allowable emission concentration in China.
3) As the amount of waste cathode carbon increases, more iron and copper could be reduced and then transferred into the metal ingot. Meanwhile, the amounts of CaF2 and NaF introduced into the slag are also increased, as a result, the slag melting point is decreased and the separation and recovery of the metal is further promoted. Upon holding in air atmosphere for 60 min first and then smelting with 14.4 wt% waste cathode carbon and 25 wt% CaO for 180 min in high purity argon atmosphere at 1450 °C, the recovery rates of Cu and Fe from copper slags reach 95.89% and 94.64%, respectively, and greater than 90% of the fluoride originated from the waste cathode carbon is transferred into the final slag. Meanwhile, the sulphur content in the obtained Fe-Cu alloy is decreased to 0.03 wt%, and the elements of Cu, C, and Fe exist in the state of mutual soluble in the metal ingot. This obtained Fe-Cu alloy (low S) can be further used as the burden to produce weathering resistant steel by electric arc furnace to replace sponge iron or scrap steel.
Contributors
MAO Kai-xuan performed the data analyses and wrote the manuscript, LI Lei performed the analysis with constructive discussions and contributed significantly to manuscript preparation, XU Miao contributed to the validation of this study.
Conflict of interest
MAO Kai-xuan, LI Lei and XU Miao declare that they have no conflict of interest.
References
[1] ZHANG Jun, QI Yuan-hong, YAN Ding-liu, XU Hai-chuan. A new technology for copper slag reduction to get molten iron and copper matte [J]. Journal of Iron and Steel Research International, 2015, 22(5): 396-401. DOI: https://doi.org/ 10.1016/S1006-706X(15)30018-2.
[2] ZHOU Xian-lin, ZHU De-qing, PAN Jian, WU Teng-jiao, Utilization of waste copper slag to produce directly reduced iron for weathering resistant steel [J]. ISIJ International, 2015, 55(7): 1347-1352. DOI: https://doi.org/10.2355/ isijinternational.55.1347.
[3] SHARMA R, KHAN R A. Sustainable use of copper slag in self compacting concrete containing supplementary cementitious materials [J]. Journal of Cleaner Production, 2017, 151: 179-192. DOI: https://doi.org/10.1016/j.jclepro. 2017.03.031.
[4] HEO J H, CHUNG Y, PARK J H. Recovery of iron and removal of hazardous elements from waste copper slag via a novel aluminothermic smelting reduction (ASR) process [J]. Journal of Cleaner Production, 2016, 137: 777-787. DOI: https://doi.org/10.1016/j.jclepro.2016.07.154.
[5] MURARI K, SIDDIQE R, JAIN K. Use of waste copper slag, a sustainable material [J]. Journal of Material Cycles and Waste Management, 2015, 17: 13-26. DOI: https://doi.org/ 10.1007/s10163-014-0254-x.
[6] GYUROV S, MARINKOV N, KOSTOVA Y, RABADJIEVA D, KOVACHEVA D, TZVETKOVA C, GENTSCHEVA G, PENKOV I. Technological scheme for copper slag processing [J]. International Journal of Mineral Processing, 2017, 158: 1-7. DOI: http://dx.doi.org/10.1016/j.minpro. 2016.11.008.
[7] SHIBAYAMA A, TAKASAKI Y, WILLIAM T, YAMATODANI A, HIGUCHI Y, SUNAGAWA S, ONO E. Treatment of smelting residue for arsenic removal and recovery of copper using pyro–hydrometallurgical process [J]. Journal of hazardous materials, 2010, 181: 1016-1023. DOI: https://doi.org/10.1016/j.jhazmat.2010.05.116.
[8] GUO Zheng-qi, ZHU De-qing, PAN Jian, ZHANG Feng. Mechanism of mineral phase reconstruction for improving the beneficiation of copper and iron from copper slag [J]. JOM, 2016, 68(9): 2341-2348. DOI: https://doi.org/10.1007/ s11837-016-2082-z.
[9] SARFO P, DAS A, WYSS G, YOUNG C. Recovery of metal values from copper slag and reuse of residual secondary slag [J]. Waste Management, 2017, 70: 272-281. DOI: https://doi.org/10.1016/j.wasman.2017.09.024.
[10] ZHANG Chang-da, HU Bin, WANG Hua-guang, WANG Ming-yu, WANG Xue-wen. Recovery of valuable metals from copper slag [J]. Mining, Metallurgy & Exploration, 2020, 37: 1241-1251. DOI: https://doi.org/10.1007/s42461-020-00224-7.
[11] GUO Zheng-qi, PAN Jian, ZHU De-qing, YANG Cong-cong. Mechanism of composite additive in promoting reduction of copper slag to produce direct reduction iron for weathering resistant steel [J]. Powder Technology, 2018, 329: 55-64. DOI: https://doi.org/10.1016/j.powtec.2018.01.063.
[12] GUO Zheng-qi, PAN Jian, ZHU De-qing, ZHANG Feng. Green and efficient utilization of waste ferric-oxide desulfurizer to clean waste copper slag by the smelting reduction-sulfurizing process [J]. Journal of Cleaner Production, 2018, 199: 891-899. DOI: https://doi.org/ 10.1016/j.jclepro.2018.07.203.
[13] SARFO P, WYSS G, MA G, DAS A, YOUNG C. Carbothermal reduction of copper smelter slag for recycling into pig iron and glass [J]. Minerals Engineering, 2017, 107: 8-19. DOI: https://doi.org/10.1016/j.mineng.2017.02.006.
[14] HEO J H, KIM B S, PARK J H. Effect of CaO addition on iron recovery from copper smelting slags by solid carbon [J]. Metallurgical and Materials Transactions B, 2013, 44(6): 1352-1363. DOI: https://doi.org/10.1007/s11663-013-9908-7.
[15] GUO Zheng-qi, ZHU De-qing, PAN Jian, YAO Wei-jie, XU Wu-qi and CHEN Jin-an. Effect of Na2CO3 addition on carbothermic reduction of copper smelting slag to prepare crude Fe-Cu alloy [J]. The Journal of The Minerals, Metals & Materials Society, 2017, 69(4): 1-8. DOI: https://doi.org/ 10.1007/s11837-017-2410-y.
[16] LI Lei, HU Jian-hang, WANG Hua. Smelting oxidation desulfurization of copper slags [J]. Journal of Iron and Steel Research International, 2012, 19(12): 14-20. DOI: https://doi. org/10.1016/S1006-706X(13)60026-6.
[17] LIU Hai-ying, WANG Jin-ling, SHEN Shi-fu, LUO You-fa. Study on process mineralogy of a used cathode of carbon block from electrolytic aluminum factory [J]. Procedia Environmental Sciences, 2012, 16: 749-757. DOI: https:// doi.org/10.1016/j.proenv.2012.10.102.
[18] WANG Jin-ling, LIU Hai-ying, LUO You-fa, NIU Qing-ren, HE Hua, SHEN Shi-fu. Study on harmless and resources recovery treatment technology of waste cathode carbon blocks from electrolytic aluminum [J]. Procedia Environmental Sciences, 2012, 16: 769-777. DOI: https://doi.org/10.1016/ j.proenv.2012.10.105.
[19] YANG Kai, ZHAO Ze-jun, XIN Xin, TIAN Zhong-liang, PENG Ke, LAI Yan-qing. Graphitic carbon materials extracted from spent carbon cathode of aluminium reduction cell as anodes for lithium ion batteries: Converting the hazardous wastes into value-added materials [J]. Journal of the Taiwan Institute of Chemical Engineers, 2019, 104: 201-209. DOI: https://doi.org/10.1016/j.jtice.2019.09.012.
[20] SHI Zhong-ning, LI Wei, HU Xian-wei, REN Bi-jun, GAO Bing-liang, WANG Zhao-wen. Recovery of carbon and cryolite from spent pot lining of aluminium reduction cells by chemical leaching [J]. Transactions of the Nonferrous Metals Society of China, 2012, 22(1): 222-227. DOI: https://doi.org/ 10.1016/S1003-6326(11)61164-3.
[21] XIE Wu-ming, ZHOU Feng-ping, LIU Jiang-yong, BI Xiao-lin, HUANG Zi-jun, LI Yu-hui, CHEN Dong-dong, ZOU Hai-yuan, SUN Shui-yu. Synergistic reutilization of red mud and spent pot lining for recovering valuable components and stabilizing harmful element [J]. Journal of Cleaner Production, 2020, 243: 118624. DOI: https://doi.org/10.1016/j.jclepro. 2019.118624.
[22] LI Xiao-ming, YIN Wei-dong, FANG Zhao, LIU Qi-hang, CUI Ya-ru, ZHAO Jun-xue, JIA Hao. Recovery of carbon and valuable components from spent pot lining by leaching with acidic aluminum anodizing wastewaters [J]. Metallurgical and Materials Transactions B, 2019, 50: 914-923. DOI: https://doi.org/10.1007/s11663-018-1485-3.
[23] ZHAO Xia, MA Lei. Hazardous waste treatment for spent pot liner [C]// 3rd International Conference on Environmental Science and Material Application. Chongqing, China: IOP Conference Series. 2018, 108: 042023. DOI: 10.1088/1755-1315/108/4/042023.
[24] OYE H A. Discussion of industrial spent pot lining treatment [C]// Proceedings of 35th International ICSOBA Conference. Hamburg, Germany. 2017: 1081-1088. https://scholar. google.com.hk/scholar?q=Discussion+of+Industrial+Spent+Pot+Lining+Treatment&hl=zh-CN&as_sdt=0&as_vis= 1&oi=scholart.
[25] GUO Zheng-qi, PAN Jian, ZHU De-qing, ZHANG Feng. Co-reduction of copper smelting slag and nickel laterite to prepare Fe-Ni-Cu alloy for weathering steel [J]. The Journal of the Minerals, Metals & Materials Society, 2018, 70: 150-154. DOI: https://doi.org/10.1007/s11837-017-2641-y.
[26] RATHORE S S, SALVE M M, DABHADE V V. Effect of molybdenum addition on the mechanical properties of sinter-forged Fe-Cu-C alloys [J]. Journal of Alloys and Compounds, 2015, 649: 988-995. DOI: https://doi.org/10.1016/ j.jallcom.2015.07.156.
[27] GB5085.3-2007. Identification standards for hazardous wastes Identification for extraction toxicity (GB5085.3-2007) in China [S]. https://www.doc88.com/p-7758295606122.html.
[28] BUSOLIC D, PARADA F, PARRA R, SANCHEZ M, PALACIOS J, HINO M. Recovery of iron from copper flash smelting slags [J]. Mineral Processing and Extractive Metallurgy, 2011, 120: 32-36. DOI: https://doi.org/ 10.1179/ 037195510X12772935654945.
[29] RUSEN A, GEVECI A, TOPKAYA Y A, DERIN B. Effects of some additives on copper losses to matte smelting slag [J]. JOM, 2016, 68(9): 2323-2331. DOI: https://doi.org/10.1007/ s11837-016-1825-1.
[30] WU Cheng-chuan, CHENG Guo-guang, TIAN Jun. A thermodynamic model for evaluation of mass action concentrations of Ce2O3-contained slag systems based on the ion and molecule coexistence theory [J]. High Temperature Materials & Processes, 2013, 32(6): 541-550. DOI: https://doi.org/10.1515/htmp-2012-0119.
[31] NAKAMOTO M, KIYOSE A, TANAKA T, HOLAPPA L, HAMALAINEN M. Evaluation of the surface tension of ternary silicate melts containing Al2O3, CaO, FeO, MgO or MnO [J]. ISIJ International, 2007, 47(1): 38-43. DOI: https://doi.org/10.2355/isijinternational.47.38.
[32] HANAO M, TANAKA T, KAWAMOTO M, TAKATANI K. Evaluation of surface tension of molten slag in multi-component systems [J]. ISIJ International, 2007, 47(7): 935-939. DOI: https://doi.org/10.2355/isijinternational.47. 935.
(Edited by FANG Jing-hua)
中文导读
以铝电解废阴极炭为添加剂熔融还原回收铜渣中的铜和铁
摘要:为了回收铜渣中的铜和铁,本文提出了一种铜渣氧化脱硫-废阴极碳熔融还原两步处理新工艺,在熔融还原过程中,废阴极碳不仅可作为还原剂,同时也可作为助熔剂降低熔渣熔点。在1450 °C时,铜渣在空气气氛中保温60 min后,添加14.4 wt%的废阴极碳和25 wt%的CaO至铜熔渣中并在高纯Ar气氛下进行熔融还原180 min,Cu和Fe的回收率分别可以达到95.89%和94.64%。同时,废阴极碳中90%以上的氟化物以CaF2和Ca2Si2F2O7形式转移至尾渣中,炉渣中可溶性F含量达到国家排放标准。此外,所得Fe-Cu合金中硫含量低至0.03 wt%。
关键词:铜渣;废阴极碳;氧化脱硫;熔融还原;铁和铜的回收;氟化物
Foundation item: Project(U1602272) supported by the National Natural Science Foundation of China
Received date: 2020-04-15; Accepted date: 2020-07-25
Corresponding author: LI Lei, PhD, Professor; Tel: +86-13987619187; E-mail: tianxiametal1008@163.com; ORCID: https://orcid.org/ 0000-0003-1205-3989

