Extraction of aluminum by pressure acid-leaching method from coal fly ash
来源期刊:中国有色金属学报(英文版)2012年第9期
论文作者:吴成友 余红发 张慧芳
文章页码:2282 - 2288
关键词:粉煤灰;铝;加压酸浸;提取率
Key words:coal fly ash; aluminum; pressure acid-leaching; extraction efficiency
摘 要:利用加压酸浸法从粉煤灰中提取铝。研究了粉煤灰粒度、硫酸浓度、反应时间、反应温度对铝提取率的影响。 通过XRD、SEM、IR对反应前、后的粉煤灰进行物相和形貌分析。确定的最佳工艺条件为粉煤灰粒度74 μm,硫酸浓度50%,反应时间4 h,反应温度180℃。在最佳工艺条件下,氧化铝的提取率为82.4%。
Abstract: Aluminum was leached out from coal fly ash by pressure acid-leaching method. The effects of coal fly ash size, sulfuric acid concentration, reaction time and reaction temperature on extraction efficiency of aluminum were investigated comprehensively. The phase and morphology of coal fly ash and solid residues after reaction were analyzed by XRD, SEM and IR. The optimal technological conditions for extracting aluminum from coal fly ash were eventually confirmed that coal fly ash with size of 74 μm and sulfuric acid with concentration of 50% are mixed in pressure reaction kettle to react for 4 h at 180 ℃. Under the optimal conditions, the extraction efficiency of aluminum can reach 82.4%.

![]()

Trans. Nonferrous Met. Soc. China 22(2012) 2282-2288
WU Cheng-you1, 2, YU Hong-fa1, 3, ZHANG Hui-fang1, 2
1. Qinghai Institute of Salt Lake, Chinese Academic of Sciences, Xining 810008, China;
2. The Graduate University of Chinese Academy of Sciences, Beijing 100049, China;
3. Department of Civil Engineering, Nanjing University of Aeronautics and Astronautics, Nanjing 210016, China
Received 27 September 2011; accepted 24 December 2011
Abstract: Aluminum was leached out from coal fly ash by pressure acid-leaching method. The effects of coal fly ash size, sulfuric acid concentration, reaction time and reaction temperature on extraction efficiency of aluminum were investigated comprehensively. The phase and morphology of coal fly ash and solid residues after reaction were analyzed by XRD, SEM and IR. The optimal technological conditions for extracting aluminum from coal fly ash were eventually confirmed that coal fly ash with size of 74 μm and sulfuric acid with concentration of 50% are mixed in pressure reaction kettle to react for 4 h at 180 ℃. Under the optimal conditions, the extraction efficiency of aluminum can reach 82.4%.
Key words: coal fly ash; aluminum; pressure acid-leaching; extraction efficiency
1 Introduction
Coal fly ash, the solid residue of coal combustion, is mainly emitted from heat-engine power station. Irregular accumulation and inappropriate disposal of coal fly ash will lead to occupancy of vast lands, and serious pollution of soil, air, water and even organism. Comprehensive utilization of coal fly ash has been studied since 1970s [1] when energy crisis, resource exhaustion, and environmental contamination have been paid more attention around the entire world. For example, coal fly ash has been used as cement concrete admixture [2-4] and water treatment agent recently [5,6]. In addition, coal fly ash, generally abundant in Al2O3 [7,8], is a kind of potential raw material for extracting aluminum. At present, bauxite, the main aluminum source for various industrial applications, is generally dependent upon import in China. Therefore, extraction of aluminum from coal fly ash is quite significant for disposing and utilizing waste materials and developing new aluminum source, and has attracted extensive attention recently.
Alkali sintering process or acid leaching method (including sulphuric acid, hydrochloric and nitric acid) has been used for extracting aluminum from fly ash. Alkali sintering process involves the following procedures: calcination of the mixture of coal fly ash and calcium oxide (or calcium carbonate or sodium carbonate) at high temperature to produce aluminates which can dissolve in sodium carbonate molten salt. Eventually, Al2O3 was attained through self-decomposition, alkali dissolution and desilication treatment, Bayer cycle process [9-11]. High extraction efficiency of aluminum can be obtained by this method. However, alkali sintering process requires high energy-consumption and produces more solid wastes, which have restricted the large-scale application of alkali sintering process in extracting aluminum from coal fly ash.
Thorough separation of silica from aluminum can be achieved by acid leaching method. However, one major shortcoming of this method is that the aluminum extraction efficiency by direct acid leaching method is obviously lower than that by alkali sinter process [12-14]. NAYAK and PANDA [15] revealed that aluminum extraction from coal fly ash at a low acid concentration and ambient temperature is not suitable for high recovery of aluminum. In order to improve the aluminum extraction efficiency, various researches have been developed. LI et al [16] provided the optimum conditions of acid leaching process with leaching temperature of 200-210 ℃, leaching time of 80 min, and volumetric ratio of acid to coal fly ash of 5:1, and the extraction efficiency of Al2O3 can reach 87%. BAI et al [17] and MATJIE et al [18] represented that the aluminum extraction efficiency by acid leaching method can be improved through adding calcium oxide (or calcium sulfate or lime) in coal fly ash and calcining the mixture at high temperature before acid-leaching reaction. BAI et al [19] also boosted the alumina extraction efficiency by sintering the mixture of coal fly ash and concentrated sulfuric acid at 300 ℃ to transform most of aluminum in coal fly ash into aluminum sulfate, which can be extracted out from coal fly ash by hot water.
In the present study, the pressure acid-leaching method was adopted to extract aluminum from coal fly ash, aiming to reduce reaction temperature and acid concentration appropriately based on high extraction efficiency in meeting energy conservation and pollution reduction targets.
2 Experimental
2.1 Materials and instruments
Coal fly ash samples in this study were collected from a heat-engine power station in the Inner Mongolia Autonomous Region, China. The sulfuric acid with the concentration of 95% (mass fraction) used in this study was of industrial grade and purchased from Zibo Chemical Factory, China. All other chemicals were of analytically grade.
High pressure reaction kettle (GS-1L) was made in Jingda Chemical Machinery, China. Both the inner container and the agitator blade of high pressure reaction kettle were specially made of pure zirconium. Ball-milling can decrease particle size and enlarge superficial area of coal fly ash, and also can break the aluminum silicon glass to some degree, which are advantageous to the reaction between sulfuric acid and coal fly ash. Pure zirconium is required as the inner container material of high pressure reactor to avoid corrosion under high pressure and strong acid. And in this study, the inner container has not been corroded obviously after all extraction experiments.
2.2 Experimental process and analytical methods
Extraction experiments of aluminum were carried out by adding 50.0 g coal fly ash, a certain amount of 95% H2SO4 and water into the high pressure reactor. The mass ratio of coal fly ash and 95% H2SO4 was fixed depending on the contents of metal oxides in coal fly ash.
Extraction of aluminum was investigated comprehensively by varying coal fly ash size (D90: 11, 38, 74, 154, 243 μm), sulfuric acid concentration (30%, 40%, 50%, 60%), reaction temperature (140, 160, 180, 200, 220 ℃) and reaction time (1, 2, 3, 4, 5 h). The temperature of exhaust and filter were fixed at 85-90 ℃ to make sure that aluminum sulfate formed after reaction does not crystallize and dissolve out at room temperature.
The milling of coal fly ash was carried out on a planet ball mill (QM-3SP2, Nanjing University, China). Solid residue of coal fly ash after leading was separated from liquid through the vacuum suction filter. The phase composition and morphology of coal fly ashes before and after leaching were analyzed by X-ray diffraction (XRD) and scanning electron microscopy (SEM). And an inductively coupled plasma-atomic emission spectrometer (ICP-AES) was used to determine the contents of aluminum in the leaching liquid to calculate the extraction efficiency, which was obtained by the following formula:
![]() (1)
(1)
where η is the extraction efficiency of aluminum, mES(Al2O3) and mCFA(Al2O3) denote the mass of Al2O3 in the leaching solution and coal fly ash before leaching, respectively.
A FTIR spectrometer was used to detect the difference of chemical composition of coal fly ashes before and after leaching under various conditions. In addition, XRF was used to determine the elemental compositions of coal fly ash and residue.
3 Results and discussion
3.1 Characterization of coal fly ash
3.1.1 Chemical composition of coal fly ash
Table 1 lists the chemical composition of coal fly ash analyzed by XRF. The content of Al2O3 in coal fly ash used in this study is 37.7% (mass fraction), rather larger than that of other metal oxides in the coal fly ash. So, this coal fly ash is obviously suitable for producing aluminum sulfate or alumina oxide.
Table 1 Chemical composition of coal fly ash used in the present work (mass fraction, %)

According to the contents of metal oxide in coal fly ash, the dosage of 95% sulphuric acid is 65.6 g based on the assumption that Al2O3, Fe2O3, CaO and MgO in coal fly ash were converted to Al2(SO4)3, Fe2(SO4)3, CaSO4 and MgSO4 after acid leaching reaction.
3.1.2 Phase composition of coal fly ash
The crystal composition of coal fly ash material used in this study can be observed from Fig. 1. The peak of mullite is very strong and the peak of free-state Al2O3 is weak, which indicates that mullite is the main form of aluminum. Figure 2 shows SEM image of coal fly ash. Round particle in SEM image may be aluminum-silica glass and mullite exists as anomalous particles [20]. Based on the analysis above, we can affirm that the uppermost existence form of aluminum is mullite and glass in coal fly ash. So conditions of pressure acid leaching must make the mullite and glassy phase decompose as soon as possible to attain high aluminum extraction efficiency.
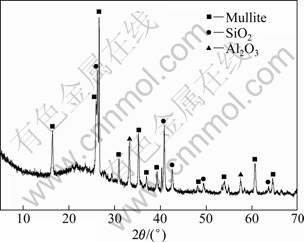
Fig. 1 XRD pattern of coal fly ash material used in this study

Fig. 2 SEM image of coal fly ash material used in this study
3.2 Influencing factors on aluminum extraction efficiency
3.2.1 Effect of coal fly ash size on extraction efficiency of aluminum
The effect of coal fly ash size on the extraction efficiency of aluminum is shown in Fig. 3. The extraction efficiency of aluminum increases as coal fly ash size (D90) decreases from 243 μm to 74 μm, but generally remains constant with coal fly ash size of 74-11 μm. The reason may be that the smaller the coal fly ash size is, the larger the specific surface area is, which is quite advantageous for the reaction between coal fly ash and sulfuric acid. However, when coal fly ash size is less than 74 μm, particle aggregation restrains the increase of surficial area. Therefore, coal fly ash of 74 μm is appropriate for the extraction of aluminum here.
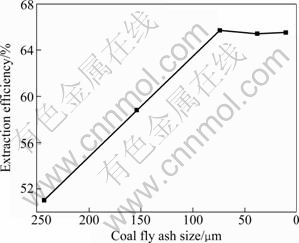
Fig. 3 Effect of coal fly ash size on extraction efficiency of aluminum (Sulfuric acid concentration 30%, reaction temperature 180 ℃ and time 4 h)
3.2.2 Effect of sulfuric acid concentration on extraction efficiency of aluminum
Figure 4 displays the effect of sulfuric acid concentration on the extraction efficiency of aluminum. The aluminum extraction efficiency increases from 65.7% to 82.4% when sulfuric acid concentration ranges from 30% to 50% (mass fraction), but dramatically diminishes to 57.8% as sulfuric acid concentration increases from 50% to 60%. Figure 5 shows XRD patterns of the leached coal fly ash. The peaks of mullite become weaker, and the characteristic peaks of calcium sulfate and quartz become sharper as the concentration of sulphuric acid raises from 30% to 50%. The change of phase composition in leached residue reveals that mullite tends to decompose at a high concentration of sulphuric acid because the contact chance between H+ and coal fly ash increases with increasing the sulfuric acid concentration. However, the peaks of mullite are weaker than those of other leached coal fly ash when the concentration of sulphuric acid increases to 60%. The phenomenon contradicts the decrease of aluminum extraction efficiency. Moreover, the residue that did not dissolve in water easily adheres to the bottom of high pressure reaction kettle after aluminum extraction reaction with sulphuric acid of 60%.
In order to study the reason why aluminum extraction efficiency decreases as sulphuric acid concentration increases to 60%, the phase composition of residue adhered to the bottom of high pressure reaction kettle was analyzed by XRD (Fig. 6). As seen from Fig. 6, the residue adhered to the bottom of high pressure reaction kettle after aluminum extraction mainly consists of Al2(SO4)3·5H2O, CaSO4, mullite and quartz. Therefore, the reason can be inferred that aluminum sulfate formed after acid-leaching reaction can not diffuse timely into the solution from reaction position and is encased by calcium sulfate, quartz and mullite because the system of 60% sulphuric acid and 50.0 g of coal fly ash powder exhibits a high-viscosity [21].
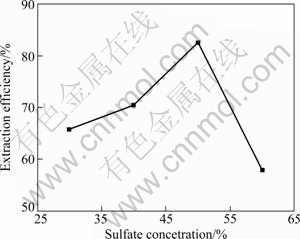
Fig. 4 Effect of sulfuric acid concentration on extraction efficiency of aluminum (Coal fly ash size 74 μm, reaction temperature 180 ℃ and time 4 h)
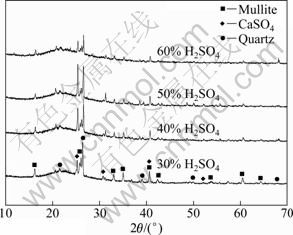
Fig. 5 XRD patterns of solid residues with different sulfuric acid concentrations (Coal fly ash size 74 μm, reaction temperature 180 ℃ and time 4 h)
3.2.3 Effect of reaction temperature on extraction efficiency of aluminum
The effect of reaction temperature on the extraction efficiency of aluminum is shown in Fig. 7. The extraction efficiency of aluminum increases from 50.2% to 85.7% as the reaction temperature increases from 140 ℃ to 220 ℃. Reaction activation energy may be great between coal fly ash and sulfuric acid according to the results in the present study and previous works [17,19] that coal fly ash begins to decompose when the temperature is higher than 150 ℃ under atmospheric pressure. NAYAK and PANDA [15] also revealed that aluminum extraction from coal fly ash at ambient temperature was not suitable for high recovery.
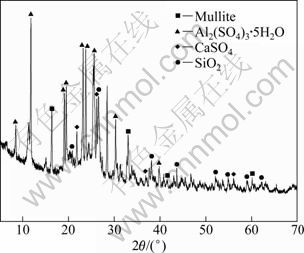
Fig. 6 XRD pattern of sediment adhered to bottom of high pressure reactor after reaction with 60% sulfuric acid (Coal fly ash size 74 μm, reaction temperature 180 ℃ and time 4 h)
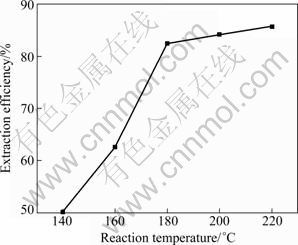
Fig. 7 Effect of reaction temperature on extraction efficiency of aluminum (Coal fly ash size 74 μm, sulfuric acid concentration 50%, and reaction time 4 h)
IR spectra of revealed coal fly ashes before and after leaching at different reaction temperatures (at 140 ℃, 160 ℃ and 180 ℃) are given in Fig. 8. IR spectrum of coal fly ash before leaching shows strong absorption bands at 1100, 893, 742, and 561 cm-1 for mullite. The leached residue shows sharp bands at 1100, 796, 673, 465 cm-1 for quartz. In addition, the adsorption bands of quartz become sharper as the reaction temperature changes from 140 ℃ to 180 ℃, which can prove that aluminum silicon glass and mullite in coal fly ash tend to decompose at high temperature.
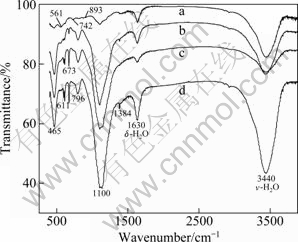
Fig. 8 IR spectra of coal fly ash before (a) and after leaching at 140 ℃ (b), 160 ℃ (c) and 180 ℃ (d)
Furthermore, the extraction efficiency increases slightly from 82.4% to 85.7% with increasing reaction temperature from 180 ℃ to 220 ℃. Therefore, 180 ℃ is considered the optimal reaction temperature taking energy consumption into account.
3.2.4 Effect of reaction time on extraction efficiency of aluminum
Figure 9 represents the effect of reaction time on the extraction efficiency of aluminum. The result shows that the extraction efficiency of aluminum rapidly increases from 32.6% to 85.9% when reaction time increases from 1 h to 5 h. The reason can be explained that aluminum- silicon glass with a low reaction activity in coal fly ash requires enough reaction time to completely decompose. Figure 10 displays SEM images of coal fly ash and solid residues after aluminum extraction reaction with different reaction time. It can be clearly observed that as reaction time extends from 1 h to 5 h, near-sphere coal fly ash particles have been gradually decomposed to flake-like, clubbed and porous particles eventually with the size of coal fly ash particle reduced to less than 10 μm. However, the increase rate of aluminum extraction efficiency decreases with extending reaction time. For example, the extraction efficiency of aluminum only increases by about 5% when the reaction time extends from 4 h to 5 h. Therefore, 4 h is considered the optimal reaction time for raising production efficiency of aluminum sulfate preparation from coal fly ash by autoclaved acid-leaching method.
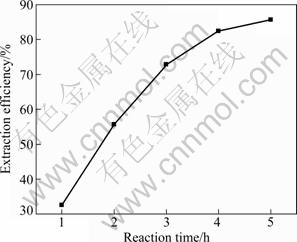
Fig. 9 Effect of reaction time on extraction efficiency of aluminum (Coal fly ash size 74 μm, sulfuric acid concentration 50% and reaction temperature 180 ℃)
3.3 Composition and mass of leached residue
As studied above, optimal conditions of pressure acid leaching method for extracting aluminum from coal fly ash with sulphuric acid figured out that coal fly ash of 74 μm and 50% sulphuric acid are mixed and react at 180 ℃ for 4 h. And aluminum extraction efficiency can reach 82.4% under this leaching condition. Elemental compositions of coal fly ash after leaching under the optimal conditions were analyzed by XRF and listed in Table 2. It is clearly indicated that aluminum is extracted into solution from coal fly ash and silica is enriched into residue after pressure acid leaching reaction.
Table 2 Composition of coal fly ashes before and after leaching (mass fraction, %)

The mass of coal fly ash after leaching decreased to 36.2 g from 50.0 g of raw coal fly ash. It shows that pressure acid-leaching method for extracting aluminum not only makes good use of coal fly ash, also reduces residues and keeps environmental contamination low compared with alkali sintering method.
4 Conclusions
1) The pressure acid leaching method was used to extract aluminum from coal fly ash, and the effects of coal fly ash size, sulfuric acid concentration, reaction temperature, and reaction time on extraction efficiency of aluminum were investigated.
2) The extraction efficiency of aluminum increases with diminishing coal fly ash size, and increasing appropriately sulfuric acid concentration, reaction temperature and reaction time.
3) The optimal technological conditions for extracting aluminum from coal fly ash have been eventually confirmed that coal fly ash with size of 74 μm and sulfuric acid with concentration of 50% are mixed in the pressure reaction kettle to react for 4 h at 180 ℃.

Fig. 10 SEM images of coal fly ash after leading for 1 h (a), 2 h (b), 3 h (c), 4 h (d), 5 h (e) (coal fly ash size 74 μm, sulfuric acid concentration 50%, and reaction temperature 180 ℃)
4) Additionally, compared with alkali sintering method, pressure acid-leaching method for extracting aluminum makes fewer residues, which is advantageous and promising for extracting aluminum from coal fly ash in a large scale production.
References
[1] AHMARUZZAMAN M. A review on the utilization of fly ash [J]. Progress in Energy and Combustion Science, 2010, 36(3): 327-363.
[2] BOUZOUBAA N, BILODEAU A, TAMTSIA B. Carbonation of fly ash concrete: Laboratory and field data [J]. Canadian Journal of Civil Engineering, 2010, 37(12): 1535-1549.
[3] YU Q J, NAGATAKI S, LIN J M, SAEKI T, HISADA M. The leachability of heavy metals in hardened fly ash cement and cement-solidified fly ash [J]. Cement and Concrete Research, 2005, 35(6): 1056-1063.
[4] JOSHI R C, LOHITA R P. Fly ash in concrete: Production, properties and uses: Advance in concrete technology [M]. Netherland: Gordon and Breach Publisher, 1997: 54-63.
[5] ALINNOR I J. Adsorption of heavy metal ions from aqueous solution by fly ash [J]. Fuel, 2007, 86(5-6): 853-857.
[6] AGYEI N M, STRYDOM C A, POTGIETER J H. The removal of phosphate ions from aqueous solution by fly ash, slag, ordinary Portland cement and related blends [J]. Cement and Concrete Research, 2002, 32(12): 1889-1897.
[7] MA Bei-yue, LI Ying, GUI Shao-gang, ZHAI Yu-chun. Preparation and sintering properties of zirconia-mullite-corundum composites using fly ash and zircon [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(12): 2331-2335.
[8] WANG Fu-yuan, WU Zheng-yan. Fly ash utilization handbook [M]. Beijing: Electric Power Press, 2004: 63-64. (in Chinese)
[9] GRZYMEK J, WARSAW, DERDAKA A. Method for obtaining aluminum oxide: USA 4149898 [P]. 1979-04-17.
[10] QIN Jian-guo. A technique of preparing silicon oxide and alumina with fly ash: China, 200710061662.X [P]. 2007-04-03. (in Chinese)
[11] PENG Fei, LIANG Kai-ming, HU An-ming. Nano-crystal glass-ceramics obtained from high alumina coal fly Ash [J]. Fuel, 2005, 84(4): 341-343.
[12] SEIDEL A, ZEMMLES Y. Mechanism and kinetics of aluminum and iron leaching from coal fly ash by sulfuric acid [J]. Chemical Engineering Science, 1998, 53(22): 3835-3852.
[13] SEIDEL A, SLUSZNY A, SHELEF G, ZIMMELS Y. Self inhibition of aluminum leaching from coal fly ash by sulfuric acid [J]. Chemical Engineering Journal, 1999, 72(3): 195-207.
[14] KELMERS A D, CANON R M, EGAN B Z, FELKER L K. Chemistry of the direct acid leach, calsinter, and pressure digestion-acid leach methods for recovery of aluminum from fly-ash [J]. Resources and Conservation, 1982, 9(1-4): 271-279.
[15] NAYAK N, PANDA C R. Aluminium extraction and leaching characteristics of Talcher thermal power station fly ash with sulphuric acid [J]. Fuel, 2010, 89(1): 53-58.
[16] LI Lai-shi, WU Yu-sheng, LIU Ying-ying, ZHAI Yu-chun. Extraction of alumina from coal fly ash with sulfuric acid leaching method [J]. The Chinese Journal of Process Engineering, 2011, 11(2): 255-258. (in Chinese)
[17] BAI Gang-hui, TENG Wei, WANG Xiang-gang, QIN Jin-guo, XU Peng, LI Peng-cheng. Alkali desilicated coal fly ash as substitute of bauxite in lime-soda sintering process for aluminum production [J]. Transaction of Nonferrous Metals Society of China, 2010, 20(s1): s169-s175.
[18] MATJIE R H, BUNT J R, VAN HEERDER J H P. Extraction of alumina from coal fly ash generated from a selected low rank bituminous South African coal [J]. Minerals Engineering, 2005, 18(3): 299-310.
[19] BAI Guang-hui, QIAO Yun-hai, BO Shen, CHEN Shuang-li. Thermal decomposition of coal fly ash by concentrated sulfuric acid alumina extraction process based on it [J]. Fuel Process Technology, 2011, 92(6): 1213-1219.
[20] BARRIOULET M, CROS H, HUSSON B, RINGOT E. Image analysis of fly ash in the characterization of the shape of the grains [C]//DIAMOND S, MINDESS S,GLASSER F R. Microstructure of Cement-based Systems/Bonding and Interfaces in Cementitious Materials. Boston, MA: Materials Research Society, 1995, 370: 125-133.
吴成友1, 2,余红发1, 3,张慧芳1, 2
1. 中国科学院 青海盐湖研究所,西宁 810008;
2. 中国科学院 研究生院,北京 100049;
3. 南京航空航天大学 土木工程系,南京 210016
摘 要:利用加压酸浸法从粉煤灰中提取铝。研究了粉煤灰粒度、硫酸浓度、反应时间、反应温度对铝提取率的影响。 通过XRD、SEM、IR对反应前、后的粉煤灰进行物相和形貌分析。确定的最佳工艺条件为粉煤灰粒度74 μm,硫酸浓度50%,反应时间4 h,反应温度180℃。在最佳工艺条件下,氧化铝的提取率为82.4%。
关键词:粉煤灰;铝;加压酸浸;提取率
(Edited by YANG Hua)
Foundation item: Project (BO210(2008)) supported by the Foundation of “Hundred Talent Program” of Chinese Academic of Sciences; Project (2008-G-158) supported by the Scientific and Technological Project of Qinghai Province, China
Corresponding author: WU Cheng-you; Tel: +86-971-6320622; E-mail: wuchengyou86@163.com
DOI: 10.1016/S1003-6326(11)61461-1

