J. Cent. South Univ. (2016) 23: 1318-1325
DOI: 10.1007/s11771-016-3182-1

Reduction mechanism of Fe2O3-Cr2O3-NiO system by carbon
ZHANG Yan-ling(张延玲), GUO Wen-ming(郭文明), LIU Yang(刘洋), JIA Xin-lei(贾昕磊)
State Key Laboratory of Advanced Metallurgy (University of Science and Technology Beijing), Beijing 100083, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract: Isothermal experiments on the reduction of Fe2O3-Cr2O3-NiO (molar ratio of Fe-to-Cr-to-Ni is 3:2:2) by graphite were carried out at 1350–1550°C, and effects of various factors on reduction degree were studied. The results show that the reaction rate of the Fe2O3-Cr2O3-NiO system is fast during the initial period (reduction degree, α<38%), and then the rate decreases until the end of the reduction. Factors such as temperature, carbon content, sample size have a more significant effect during the final stage (α>38%). The metallic product formed at the initial stage (a Fe-Ni alloy) greatly promotes the reduction of Cr2O3 at the final stage. Further, during the reduction of Fe2O3-Cr2O3-NiO by carbon, interfacial reaction is the rate-controlling step and g(α)=1-(1-α)0.5 is the reaction mechanism for the initial stage, whereas two-dimensional diffusion is the rate-controlling step and f(α)=α+(1-α)ln(1-α) is the reaction mechanism for the final stage. The apparent activation energies are 55.43 kJ/mol and 174.54 kJ/mol for the initial and the final stages, respectively.
Key words: Fe2O3-Cr2O3-NiO system; isothermal reduction; reduction degree; kinetics; reaction mechanism
1 Introduction
With the rapid development of the stainless steel industry in China, large amounts of byproducts such as steel dust, slag, and pickling sludge are being generated. Studies have shown that 18-33 kg/t of dust is generally produced during the stainless steel-making process. The Fe, Cr and Ni contents are 40%-60%, 8%-15%, and 3%-9% (mass fraction), respectively [1-2], and these metals are present mainly in the form of oxides [3-5]. To effectively control the recovery of these metals, it is essential to understand the reduction behavior of such multi-metal systems and how the metallic elements interact with each other.
Previous studies on the reduction of Cr2O3 or chromite by carbon [6-8] and other reductants [9-12] have reported the effects of factors such as temperature, sample amount, and reductant content on the reduction process. PENG et al [7] simulated the reduction of Cr2O3 in dust by using Cr2O3, carbon, CaO and iron powder as the raw materials and suggested that, in the initial stage, the reduction process was probably controlled by a chemical reaction, whereas it was determined by the diffusion of carbon in the final stage. ZHANG et al [13] investigated the thermodynamic principle of preparation of Cr3C2 and Cr with Cr2O3 and C as raw materials, and found that there are two principles for Cr2O3 reduced by carbothermal method, one reaction depends mainly on CO/CO2 gas-solid transmitted and another one depends mainly on the solid-solid reaction between carbon and chromium oxide. The latter is dominant when Cr2O3 and C contact closely. LI et al [14] found that the fast reduction of carbon-bearing chromite pellet can be achieved above 1300 °C, the reduction reaction of iron is easier than that of chromium and the mole ratio of C-to-O=1.2 is better for the prereduction of chromite pellete. LI et al [15] found that most of iron oxide was reduced while chromium oxide remained as the optimal amount of reducing agent added at 1100 °C. The reduction rate of iron increases with decreasing the granularity of core sample. WENZEL et al [16] employed aluminium as reductant to reutilize chromium oxide contained in waste ashes, showing that the utilization of Fe2O3 is the iron source with a 30% excess of the stoichiometric amount of Al and no chromic acid addition could obtain a chromium conversion of (76.8±12.3)%. MA et al [17] studied the non-isothermal reduction mechanism of Fe2O3-NiO-Cr2O3 mixture and found that the reduction of Fe2O3 and Cr2O3 was influenced by the formation of FeCr2O4 and that the final products were a series of solid solutions that mainly contained (Fe, Ni), (Fe, Cr), and small amounts of carbides. Moreover, direct reduction was the reaction mechanism of the reduction process.
The present investigation is to study the reduction of mixtures of Fe, Cr and Ni oxides coexisting in a complicated system. Also, the effects of factors such as temperature, initial carbon content, and pellet size on the reduction degree and reaction rate are discussed. Kinetic information such as activation energy and reaction mechanism is obtained.
2 Experimental procedures
2.1 Materials
The chemical reagents iron oxide(Fe2O3), chromium oxide(Cr2O3), and nickel oxide(NiO) were employed as the raw materials. Graphite carbon (purity higher than 99.85%) was used as the reductant in the experiments. Argon gas with a purity of 99.99% was used as the inert atmosphere. A high-purity aluminum crucible (outer diameter of 28 mm, inner diameter of 24 mm, height of 50 mm) was used as the container. For each sample, the molar ratio of Fe, Cr and Ni was set as 3:2:2, and the initial carbon content depended on the experiment. The blend was thoroughly mixed in a porcelain mortar to ensure homogeneity and good distribution, and then cylindrical compacts sized d16 mm×20 mm and d10 mm×10 mm were formed under pressure of 40 MPa for 2 min. The experimental schedule is shown in Table 1.
2.2 Apparatus and procedure
The apparatus used was a molybdenum-wired resistive furnace equipped with an ammonia cracking furnace, a temperature-control instrument, a water- cooling system, a balance with a detection precision of 1 mg and a data-collection program. The temperature in the tube was measured with Pt30%Rh-Pt6%Rh thermocouples. The schematic illustration of the apparatus is presented in Fig. 1.
In a typical experiment, argon gas was passed through the furnace tube at a high rate to drive out the air before heating, and the gas flow rate was maintained at 1 L/min during the experiment. After the temperaturereached the target value, the high-purity aluminum crucible containing the pellet sample was placed in the upper zone of the reaction tube for approximately 3 min by hanging the crucible from the hook of the balance and then moving the crucible to the reaction zone of the tube; this moment was set as the beginning time (0 min) of the reduction. During the holding time, the mass loss of the pellet was continuously recorded by the data-acquisition system. After completing the experiment, the sample was cooled in Ar atmosphere and the data-acquisition system was turned off.
2.3 Analysis
The reduction degree (α) is given by
 (1)
(1)
where Δm (g) and m (g) are the mass loss of the pellet and the mass of the total initial oxygen in the sample, respectively. Here, it is assumed that the oxygen was removed mainly in the form of CO because CO2 is thermodynamically unstable under temperatures higher than 1300 °C.
The characterization of the reduction products was identified by X-ray diffraction technique (XRD, 21 kW, MAC Science, Japan). The morphology and component distribution of the samples at a specific reaction time were observed and analyzed by a scanning electron microscope coupled with an energy dispersive spectrometer (SEM–EDS, JSM-6510, 25 kV, JEOL, Japan).
3 Results and discussion
3.1 Effects of various factors on reduction of Fe2O3- Cr2O3-NiO system
3.1.1 Effect of temperature
Figure 2 shows the effect of temperature on the reduction of the Fe2O3-Cr2O3-NiO mixture for molar ratios of carbon to oxygen (nC:nO) of 1.0. As can be seen in Fig. 2(a), the reduction degree obtained is more than 93% when the temperature is higher than 1350 °C. On the other hand, the reduction degree is much lower and the time needed for complete reduction is longer at 1350 °C. For each reduction curve, the reduction degree increases linearly and then decreases somewhat, finally remaining unchanged until the end of the experiment. It can be learned that the initial reduction degree remained nearly constant for each temperature when the reduction degree is less than 38%. The reduction of NiO and Fe2O3 is thermodynamically very easy to perform [17]. Calculations show that the reduction degree should be 11.76% if the NiO in the mixture is reduced completely and 64.71% if both NiO and Fe2O3 are reduced. Therefore, it is inferred that at this stage, the main activity tends to be the reduction of Ni and some Fe from their respective oxides, along with the formation of a liquid Fe-Ni solution. When the reduction degree is above 38%, it is suggested that temperature has a profound effect on the reduction degree as seen in Fig. 2. The higher the temperature, the shorter the time needed to reach the maximum reduction degree. It also reveals that the reduction of Cr from its oxide or from the complex oxides combined with Fe (such as FeCr2O4) occur mainly in this period. The corresponding relationships between the reaction rate and time (dα/dt) (see Fig. 2(b)) show that the maximum reaction rate increases with increasing temperature and the reaction rate for α<38% (within 4 min) is much higher than that for α>38% (after 4 min).
Table 1 Experimental schedule for isothermal reduction
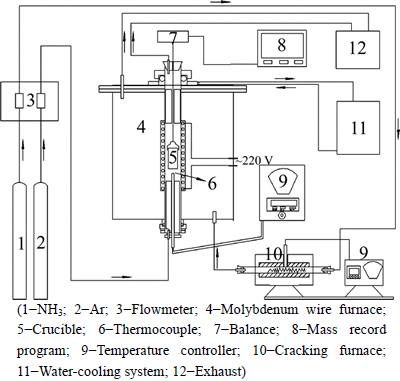
Fig. 1 A schematic illustration of experimental apparatus

Fig. 2 Effect of temperature on reduction degree (a) and reaction rate for nC:nO of 1.0 (b)
3.1.2 Effect of reductant (carbon) content
Figure 3 shows the relationship between the reduction degree and time for various carbon contents at 1550 °C. The final reduction degree of the Fe2O3-Cr2O3- NiO-C mixture increases from 48.12% to 96.00% as nC:nO increases from 0.5 to 1.0. Once the carbon content exceeds the stoichiometric quantity required (nC:nO=1.0), the final reduction degree tends to remain unchanged. Thus, the reduction degree of a mixture system initially increases with increasing carbon content and then remains unchanged.
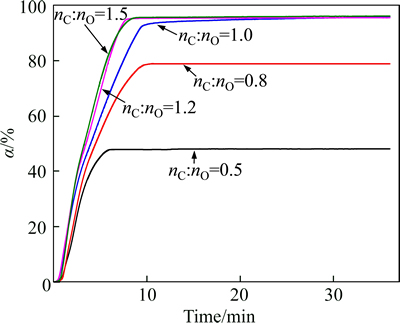
Fig. 3 Effect of reductant content on reduction degree (T= 1550 °C)
It is noted that the curves almost coincide when nC:nO equals 1.2 and 1.5. These results indicate that the reduction process is no longer under the influence of carbon content once the carbon content is sufficient (nC:nO>1.0). On the other hand, the results of the XRD analysis described in subsequent sections indicate that the reduction process of Cr2O3 is from oxide to carbide to metal. This process causes some dissolution of carbon into the Fe–Cr–Ni–C melt and some of the carbon is consumed by carbide formation. Therefore, the final reduction degree of mixture could not reach 100%, even though the carbon content is sufficient.
A similar tendency is apparent in the reduction curves of various carbon contents, which indicates that the carbon content in the mixture has little effect on the reaction rate. The carbon content only has some effects on improving the final reduction degree of the mixture.
3.1.3 Effect of pellet size
Figure 4 shows the effect of pellet size on the reduction degree at 1450 °C. It is found that the two different-sized pellets have the same final reduction degree (97%). Thus, pellet size has no effect on the final reduction degree of the system. However, the size does have a significant influence on the reaction rate. The reaction rate of the small pellet is faster than that of the large pellet throughout the whole reduction process. The investigation on the reduction of Cr2O3/carbon pellets previously reported [7] has the same results. The same carbon content and experimental temperature result in the same thermodynamic reduction of each pellet. Hence, pellets of different sizes have the same final reduction degree. However, dimensional changes caused changes in kinetic behavior. As compared to the small pellet, much more product is generated for the large pellet, forming a thick product layer and increasing the diffusion distance for reactants to contact and causing a decrease in the reaction rate. Hence, it takes more time for complete reduction of the large pellet.
From Figs. 2-4, it is observed that a reduction degree below 38% exhibits different tendencies as compared with that at 38% and higher. The reaction rate is faster when the reduction degree is below 38%. At reduction degrees of 38% and higher, the reaction rate decreases until the end of the reaction. Below 38%, the reduction degree is much less affected by factors such as temperature, carbon content and sample size. Investigations [7, 18] have also suggested that the reduction process of some oxides such as chromium oxide or chromite by carbon tends to be controlled by different mechanisms at different stages. In the present work, the reduction process of Fe2O3-Cr2O3-NiO is divided into two stages at reduction degree of 38%.

Fig. 4 Effect of pellet size on reduction degree of oxide system (T=1450 °C, nC:nO =1.0)
3.2 Comparison between different systems
In order to examine the behaviors of Cr2O3 and NiO in the mixture, carbothermic reductions of Fe2O3- Cr2O3-C and Fe2O3-NiO-C systems are also conducted. The reduction curves of the three systems at 1450 °C are given in Fig. 5(a), and the corresponding instantaneous reaction rates (dα/dt) are shown in Fig. 5(b). It is found that the final reduction degree of the Fe2O3-Cr2O3-NiO-C mixture is 97%, which is the highest of the three systems, and thus the final reduction degree for the three systems is Fe2O3-Cr2O3-NiO-C>Fe2O3-Cr2O3-C>Fe2O3-NiO-C. On the other hand, the time required to reach the final reduction degree is Fe2O3-NiO-C2O3-Cr2O3- NiO-C< Fe2O3-Cr2O3-C.
For the Fe2O3-NiO-C mixture, Fig. 5(a) suggests that the metal product, defined as a dense Fe-Ni alloy phase, is formed early in the initial period. This tends to limit the further diffusion of carbon, and thus limits the final reduction degree. Research on the reduction of NiFe2O4 [19] shows a similar tendency, during which incomplete reduction (about 80%) of NiFe2O4 by hydrogen at 900-1100 °C is observed. The data suggest that this incomplete reduction could be attributed to the formation of a dense metallic layer (FexNiy) surrounding the wüstite layer, which prevents the further diffusion of the products.

Fig. 5 Comparison of reduction curves (a) and instantaneous reaction rates (b) of different systems
Figure 6 shows the XRD patterns of the Fe2O3-Cr2O3-C mixture at different reaction times. It is found that the transformation of Fe2O3 to Fe3O4 and the generation of FeCr2O4 are the major processes that occur in the first 6 min. FeCr2O4 is then reduced to metallic Fe, releasing Cr2O3. Metallic Cr (in the Fe-Cr alloy) appears at 15 min, which suggests that FeCr2O4 goes through an extended process in order to generate the metallic product, resulting in a slower reaction rate for the Fe2O3-Cr2O3-C mixture. The XRD patterns of the Fe2O3-Cr2O3-NiO-C mixture over time are shown in Fig. 7. It can be seen that metallic product (Fe, Ni) is generated in the first 6 min through the reduction of Fe2O3 and NiO. A higher final reduction degree and shorter time to reach that reduction degree are observed in the Fe2O3-Cr2O3-NiO-C mixture as compared with the Fe2O3-Cr2O3-C system. This could be explained by the fact that the dissolution of chrome into the Fe-Ni-C alloy can decrease the activity of Cr and greatly promote the reduction of Cr2O3. Also, the appearance of a liquid phase (Fe-Ni-C) tends to improve kinetic conditions.
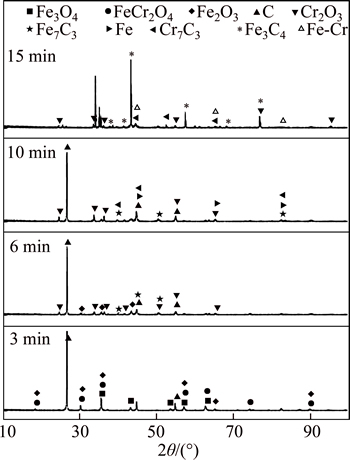
Fig. 6 X-ray diffraction patterns of reduction product of Fe2O3-Cr2O3 mixture at different time
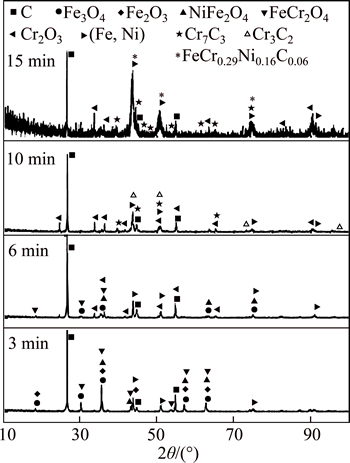
Fig. 7 X-ray diffraction patterns of reduction products of Fe2O3-Cr2O3-NiO mixture at different times
It is noted that the reduction curve of the Fe2O3-Cr2O3-NiO-C system at the initial stage coincides with the curve of the Fe2O3-NiO-C system, and the curve at the final stage parallels the curve of the Fe2O3-Cr2O3-C system. Also, as shown in Fig. 5(b), the reduction rates of the Fe2O3-Cr2O3-NiO-C and Fe2O3-NiO-C systems are the same at the beginning of the reduction process and equal to that of the Fe2O3-Cr2O3-C system at 7-12 min. From the comparison of the three systems, it is concluded that the reduction of Fe2O3 and NiO is the major reaction of the Fe2O3-Cr2O-NiO mixture at the initial stage and that the reduction of Cr2O3 occurs at the final stage. The presence of Cr2O3 has no influence on the reduction of the Fe2O3 and NiO at the initial stage. However, the metallic product of the initial stage can promote the reduction of Cr2O3 and complex oxides at the final stage, which significantly increases the reduction degree of the Fe2O3-Cr2O3-NiO-C system.
3.3 Mechanism of carbothermic reduction of Fe2O3- Cr2O3-NiO-C system
As mentioned above, the reduction mechanism of the Fe2O3-Cr2O3-NiO-C system at the initial stage is different from that at the final stage. As can be seen in Fig. 7, the intensity of carbon and the oxides decreases with increasing reaction time. Shortly after the beginning of reduction, Cr2O3 and some NiO and Fe2O3 transform into complicated oxides, such as FeCr2O4 and NiFe2O4. NiFe2O4 is reduced to Fe and Ni, and a (Fe, Ni) alloy is formed from the metallic product at 6 min. The intensity of the (Fe, Ni) alloy increases at this period. The reduction of Cr2O3 begins after 6 min. Moreover, the reduction process proceeds in the order of FeCr2O4→ Cr2O3→chromium carbide. Then, Cr is dissolved into the (Fe, Ni) alloy and the Fe-Cr-Ni-C alloy is formed.
During the carbothermic reduction of the Fe2O3-Cr2O3-NiO-C system, the following schematic reduction reactions are thought to take place:
Fe2O3+2Cr2O3+C=2FeCr2O4+CO, △GΘ=-0.17T-22.11 (2)
NiO+Fe2O3=NiFe2O4,
△GΘ=0.0061T-20.37 (3)
NiO+C=Ni+CO, △GΘ=-0.17T+74.09 (4)
3Fe2O3+C=2Fe3O4+CO, △GΘ=-0.23T+74.54 (5)
NiFe2O4+C=Ni+Fe2O3+CO,
△GΘ=-0.18T+92.90 (6)
 Fe2O3+C=
Fe2O3+C= Fe+CO, △GΘ=-0.17T+116.37 (7)
Fe+CO, △GΘ=-0.17T+116.37 (7)
FeCr2O4+C=Fe+Cr2O3+CO,
△GΘ=-0.17T+183.30 (8)
 Cr2O3+C=
Cr2O3+C=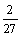 Cr7C3+
Cr7C3+ CO,
CO,
△GΘ=-0.14T+153.62 (9)
 Fe3O4+C=
Fe3O4+C=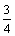 Fe+CO, △GΘ=-0.16T+114.84 (10)
Fe+CO, △GΘ=-0.16T+114.84 (10)
At the initial stage, the oxides are in close contact with carbon. Solid-state reduction is the major reaction. And a theoretical calculation reveals that the Gibbs energy for the reaction between Cr2O3 and CO is 134.363 kJ/mol when 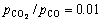 at 1500 °C. Thus, the reaction between Cr2O3 and CO rarely occurs under experimental conditions. Therefore, solid carbon or carbon dissolved in the (Fe, Ni) alloy is the reductant at the final stage for the reduction of Cr2O3.
at 1500 °C. Thus, the reaction between Cr2O3 and CO rarely occurs under experimental conditions. Therefore, solid carbon or carbon dissolved in the (Fe, Ni) alloy is the reductant at the final stage for the reduction of Cr2O3.
3.4 Reaction kinetics of Fe2O3-Cr2O3-NiO-C system
Figures 8(a) and (b) show the SEM images of samples reduced at 1450 °C at 3 min and 6 min, respectively, together with the line distributions of C, O, Cr, Fe and Ni. The oxides are in close contact with the reductant in 3 min, and the reduction happens at the interface of oxides and carbon. Hence, the reaction rate depends on the rate at which the oxides react with the carbon. As the reaction progresses, the oxides and the carbon are separated by the product layer, which is a zone with high metallic elements concentration and lowoxygen content (see in Fig.8(b)). The reactants should diffuse through the layer for further reduction. Consequently, it is predicted that this solid-state diffusion tends to be the rate-controlling step at the final stage.

Fig. 8 SEM photomicrographs of Fe2O3-Cr2O3-NiO-C sample reduced under 1450 °C at 3 min (a) and 6 min (b)
Accordingly, the calculations by using corresponding kinetic models [20] for the initial and final stages were performed. The curves under different temperatures are shown in Fig. 9. These curves can satisfy a linear relationship well at the initial and the final stage. Therefore, for carbothermic reduction of the Fe2O3-Cr2O3-NiO system, an interfacial reaction is the rate controlling step and g(α)=1-(1-α)0.5 is the reaction mechanism for the stage of reduction degree below 38%, whereas two-dimensional diffusion is the rate-limitation step and f(α)=α+(1-α)ln(1-α) is the reaction mechanism for the final stage.
The apparent activation energy (Ea) of reduction is calculated from the Arrhenius equation:
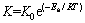 (11)
(11)
where K0 is the frequency factor; Ea is the apparent activation energy; R is the gas constant and T is the thermodynamic temperature.
The relationships between the logarithm of the reaction rate (lnK) and the absolute temperature (1/T) at both stages are plotted and presented in Fig. 10. The calculated values of the apparent activation energy are 55.43 kJ/mol and 174.54 kJ/mol for the initial and the final stage, respectively.
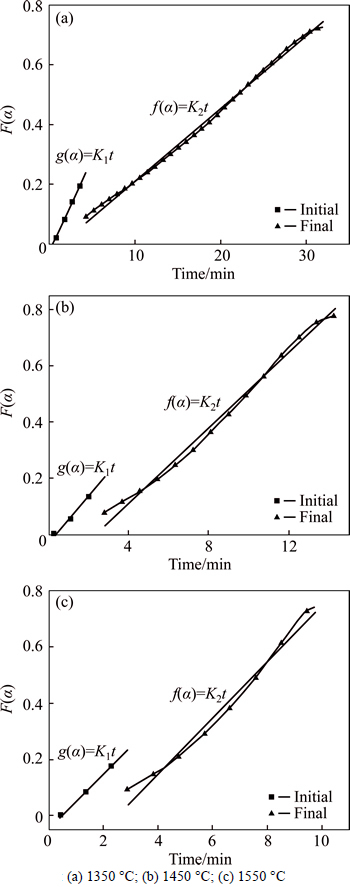
Fig. 9 Model calculation at various temperatures of Fe-Cr-Ni-O system (nC:nO=1.0):
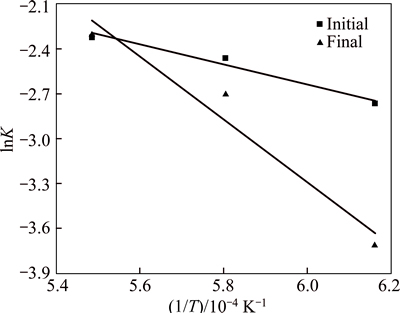
Fig. 10 Arrhenius plots for initial and final stages of Fe-Cr-Ni-O system reduction
4 Conclusions
Isothermal experiments are conducted on the carbothermic reduction of Fe2O3-Cr2O3-NiO mixtures, and the results are as follows:
1) The reaction rate is fast at the initial period (α<38%), and then it decreases until the end of the reaction. Temperature, carbon content, sample size have a much greater effect on the final reduction process (α>38%).
2) The final reduction degree increases in the order of Fe2O3-NiO2O3-Cr2O32O3-Cr2O3-NiO, and the time required to complete the reaction decreases in the order of Fe2O3-Cr2O3-C>Fe2O3-Cr2O3-NiO-C>Fe2O3- NiO-C. The metallic product (a Fe-Ni alloy) formed at the initial stage promotes the reduction of Cr2O3.
3) For the reduction of Fe2O3-Cr2O3-NiO by carbon, an interfacial reaction is the rate-controlling step and g(α)=1-(1-α)0.5 is the mechanism for the initial stage, whereas two-dimensional diffusion is the rate-controlling step and f(α)=α+(1-α)ln(1-α) is the mechanism for the final stage. The apparent activation energies are 55.43 kJ/mol and 174.54 kJ/mol for the initial and the final stage, respectively.
References
[1] SONG Hai-chen, PENG Bing. Present situation of comprehensive utilization and research activity of stainless steelmaking dust [J]. Multipurpose Utilization of Mineral Resources, 2004(3): 18-22. (in Chinese)
[2] MA Guo-jun, FAN Wei, XU Zhi-hao, XUE Zheng-liang, WANG Wei, SU Wei-hou. Distribution behavior of chromium and other elements in the stainless steel plant dust [J]. The Chinese Journal of Process Engineering, 2010, 10(s1): 68-72. (in Chinese)
[3] TANG Mo-tang, PENG Ji, PENG Bing, YU Di, TANG Chao-bo. Thermal solidification of stainless steelmaking dust [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(1): 202-206.
[4] ZHANG Huai-wei, HONG Xin. An overview for the utilization of wastes from stainless steel industries [J]. Resources, Conservation and Recycling, 2011, 55(8): 745-754.
[5] WEI Fen-rong, ZHANG Yan-ling, WEI Wen-jie, YANG Xiao-gang. Chemical composition of dust from stainless steel smelting and existing forms of Cr and Ni [J]. The Chinese Journal of Process Engineering, 2011, 11(5): 786-793. (in Chinese)
[6] MORI T, YANG J, KUWABARA M. Mechanism of carbothermic reduction of chromium oxide [J]. ISIJ International, 2007, 47(10): 1387-1393.
[7] PENG Bing, SONG Hai-chen, WANG Jia, CHAI Li-yuan, WANG Yun-yan, MIN Xiao-bo. Reduction process of Cr2O3/carbon pellets [J]. Journal of Central South University: Science and Technology, 2005, 36(4): 571-575. (in Chinese)
[8] CHEN Jin, ZHAO Jing, ZHANG Meng, YAN Hong, ZHOU Jian-xiong. Carburization of ferrochromium metals in chromium ore fines containing coal during voluminal reduction by microwave heating [J]. Journal of Central South University of Technology, 2009, 16(1): 43-48.
[9] WEI Guang-ye, QU Jing-kui, ZHENG Yu-dong, QI Tao, GUO Qiang. Preparation of Cr2O3 precursors by hydrothermal reduction in the abundant Na2CO3 and Na2CrO4 solution [J]. International Journal of Minerals, Metallurgy and Materials, 2012, 19(11): 978-985.
[10] QIAN Yue-jin, JIANG Ming-xue, LI Liu-sheng. Effect of atmosphere on sintering of high Cr2O3 bearing refractories considered from thermodynamic calculation [J]. China’s Refractories, 2009, 18(4): 22-26.
[11] LIU Wei-dong. Study and practice of reutilization of dust retracted from stainless steel refining [J]. Steelmaking, 2011, 27(6): 66-69. (in Chinese)
[12] QIAN Yue-jin, JIANG Ming-xue. Analysis the sintering mechanism of Cr2O3 at hydrogen atmosphere from thermodynamic calculation [J]. Journal of Wuhan University of Technology, 2009, 31(20): 47-49. (in Chinese)
[13] ZHANG Xue-feng, FANG Min-xian, TONG Yan-wei, LI Hui-rong. Study on thermodynamic principle of preparation of Cr3C2 and Cr by carbothermic reduction [J]. Materials Review, 2010, 24(9): 108-111. (in Chinese)
[14] LI Jian-chen, BAI Guo-hua, LI Guang-hui. Solid-state reduction properties of carbon-bearing chromite pellets [J]. The Chinese Journal of Nonferrous Metals, 2011, 21(5): 1159-1164. (in Chinese)
[15] LI Cheng, CHANG Guo-hua, PENG Jing-xing. Selective reduction of chromite fines by microwave treatment [J]. The Chinese Journal of Nonferrous Metals, 2013, 23(2): 503-509. (in Chinese)
[16] WENZEL B M, ZIMMER T H, FERNANDEZ C S, MARCILIO N R, GODINHO M. Aluminothermic reduction of Cr2O3 contained in the ash of thermally treated leather waste [J]. Brazilian Journal of Chemical Engineering, 2013, 30(1): 141-154.
[17] MA P, LINDBLOM B, BJ RKMAN B. Mechanism study on solid-state reduction in the Fe2O3-NiO-Cr2O3-C system using thermal analyses [J]. Scandinavian Journal of Metallurgy, 2005, 34(1): 22-30.
RKMAN B. Mechanism study on solid-state reduction in the Fe2O3-NiO-Cr2O3-C system using thermal analyses [J]. Scandinavian Journal of Metallurgy, 2005, 34(1): 22-30.
[18] DOLLY C, RANGANATHAN S, SINHA S N. Investigations on the carbothermic reduction of chromite ores [J]. Metallurgical and Materials Transactions B, 2005, 36(4): 437-444.
[19] MOHAMED H K. Isothermal reduction kinetics at 900-1100 °C of NiFe2O4 sintered at 1000-1200 °C [J]. Journal of Analytical and Applied Pyrolysis, 2005, 73(1): 123-129.
[20] LUO Shi-yong, ZHANG Jia-yun, ZHOU Tu-ping. Models for kinetic analyses of solid-solid reactions and their applications [J]. Materials Review, 2000, 14(4): 6-7. (in Chinese)
(Edited by FANG Jing-hua)
Foundation item: Project(51074025) supported by the National Natural Science Foundation of China; Project(FRF-SD-12-009A) supported by the Fundamental Research Funds for the Central Universities, China
Received date: 2014-12-15; Accepted date: 2015-04-03
Corresponding author: ZHANG Yan-ling, PhD, Associate Professor; Tel: +86-10-82375191; E-mail: zhangyanling@metall.ustb.edu.cn