文章编号: 1004-0609(2004)12-2114-06
水杨酸锰的热分解机理及纳米氧化锰形貌
李良超, 郝仕油, 林秋月
(浙江师范大学 化学系, 金华 321004)
摘 要: 用流变相-前驱物反应法合成了水杨酸锰配合物。 通过元素分析和TG分析确定该配合物的组成为Mn(HSal)2·2.5 H2O(HSal=o-OHC6H4COO); XRD确定该配合物为单斜晶系, 晶胞参数为a=1.8935(4) nm, b=0. 6179(0) nm, c=1.7325(6) nm, β=114.93(1)°, V=1.8382(3) nm3, Z=6, ρcal=1.775kg/dm3, ρexp=1.769kg/dm3; IR研究表明配合物中羧酸根与Mn2+以双齿螯合方式配位。 样品在氮气中经330℃热分解的最终产物是MnO和有机化合物, 在空气中经800℃热分解的最终产物为Mn3O4。 TEM观察表明, MnO和Mn3O4均为球形粒子, 平均粒径分别约为25和28nm; 激光粒度分析仪测定MnO和Mn3O4的平均粒径约为45.6和69.1nm。
关键词: 水杨酸锰; 流变相; 热分解; 纳米氧化锰
中图分类号: O646 文献标识码: A
Thermal decomposition mechanism of
manganese salicylate and shape of nanometric manganese oxide
LI Liang-chao, HAO Shi-you, LIN Qiu-yue
(Department of Chemistry, Zhejiang Normal University, Jinhua 321004, China)
Abstract: Manganese salicylate was synthesized with the rheological phase reaction method. The composition is Mn(HSal)2·2.5 H2O(HSal=o-OHC6H4COO) through elemental and TG analysis. This complex belongs to monoclinic system with cell dimension: a=1.8935(4) nm, b=6.1790(2) nm, c=17.3256(1) nm, β=114.93(1)°, V=1.8382(3) nm3, Z=6, ρcal=1.775kg/dm3, ρexp=1.769kg/dm3 through powder X-ray diffraction. IR spectrum of sample shows that there is an asymmetric bidentate bridging coordination between the COO- and the Mn2+ of manganese salicylate. Final products of sample thermal decomposition are MnO powder and organic compounds in N2 at 330℃ and Mn3O4 in air at 800℃. TEM measurement indicates that both MnO and Mn3O4 are spherical particles. The average diameter of the MnO and Mn3O4 particles are 25nm and 28nm through TEM and 45.6nm and 69.1nm through laser particles size analyzer.
Key words: manganese salicylate; rheological phase; thermal decomposition; nano-manganese oxide
纳米MnOx由于其尺寸小、 比表面积大, 因而具有与常规材料和单个原子、 分子不同的独特性质, 在电极材料、 催化材料和陶瓷材料等领域的应用引起了科学工作者的高度重视[1-4]。 迄今为止, 制备纳米MnOx的方法主要有溶胶法、 溶胶-凝胶法、 微乳法、 固相氧化还原合成法和激光蒸凝法[5-7]。 本文作者曾用流变相反应法合成了具有新型结构的过渡金属水杨酸盐[8-12], 并通过热分解实验制得了相应的金属氧化物超微粉体和用一般合成方法难以得到的有机化合物。 本文作者以水杨酸和碳酸锰为原料用流变相反应法合成了水杨酸锰配合物, 通过XRD、 TG和IR确定了水杨酸锰的组成、 晶体结构、 金属离子与羧酸根的配位方式, 通过定温热分解实验得到了MnO和 Mn3O4纳米粉体, 用TEM观察了该纳米粒子的形貌和粒径, 用激光粒度分析仪测定了它们的粒度分布, 详细地探讨了水杨酸锰的热分解机理。
1 实验
1.1 样品的制备
实验原料和辅料均为分析纯试剂。
称取摩尔比为1∶2.05的碳酸锰和水杨酸, 混合研磨均匀。 按文献[10]的方法加适量的去离子水调制成流变体, 置于聚四氟乙烯反应器(带不锈钢外套)内于70℃下反应12h, 将产物用乙醇洗涤3次, 50℃真空干燥至恒重, 得到棕色粉末样品。
将该样品分别于330℃(氮气中)和800℃(空气中)保温2h, 其固相产物分别为灰绿色的MnO和灰色的Mn3O4粉末。
1.2 组成和结构特性分析
样品的碳和氢的含量用Perkin-Elmer 240B型元素分析仪测定, 用EDTA滴定法测定样品中Mn2+的含量(以甲基百里酚蓝为指示剂, 六次甲基四胺作缓冲液), 以环己烷-排液法测定样品的密度。 用Philps-Pw 3040/60型粉末X射线衍射仪测定前驱物及其热分解固相物的粉末衍射数据, 测试条件为: 石墨单色器滤波, 旋转阴极铜靶Ka1辐射(λ=0.154056nm), 管电压40kV, 管电流40mA。 在Shimadza FT-IT800型傅里叶变换红外光谱仪上, 采用KBr压片法, 在4000~400cm-1范围内记录样品红外光谱。 用瑞士Metter Toledo TGA/SDTA 851e热分析仪在氮气(50mL/min)中以20℃/min的升温速率(温度范围为20~900℃)测定样品的TG和DTA曲线。 依据TG和DTA曲线的数据, 在管式炉中氮气气氛下经330℃分解试样3~5g, 分解反应产物按文献[12]的方法收集。 对收集的产物进行红外光谱分析, 并用VG Analytical7070E-HF型色谱-质谱联用仪测定热分解气相凝聚物的色谱及各组分的质谱。 用JTM 100 SX透射电子显微镜获得在氮气气氛中330℃样品热分解固相残留物MnO的照片和空气条件下800℃样品热分解固相残留物Mn3O4的照片。 用MASTERSIZER 2000型(0.02~2000μm)激光粒度分析仪(水为分散剂)测定MnO和Mn3O4的粒度分布曲线。
2 结果和讨论
2.1 水杨酸锰的元素含量及组成
水杨酸锰样品的元素含量(质量分数)的测定值(括号内为计算值)为: Mn 14.46%(14.69%), C 45.38%(44.96%), H 4.32%(4.14%)。 结合热重分析数据可以确定样品的组成为Mn(HSal)2·2.5H2O(HSal=o-OHC6H4COOH)。
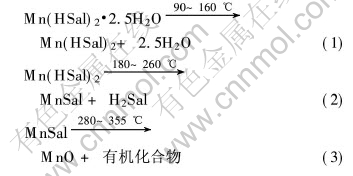
图1所示为样品在氮气中的TG和DTA曲线。 TG曲线有3个阶段, 第一阶段为90~160℃, 质量损失为11.86%, 相当于失去2.5摩尔结晶水(12.04%); 第二阶段为180~260℃, 质量损失为37.10%, 相当于由Mn(Hsal)2生成MnSal(36.93%); 第三阶段为280~355℃, 质量损失为31.74%, 相当于由MnSal生成MnO(32.08%), 其差值也许是由于在氮气氛中产生少量结碳所致。
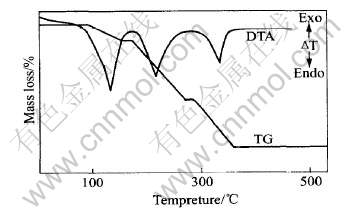
图1 Mn(HSal)2·2.5H2O的TG和DTA曲线
Fig.1 Curves of TG and DTA for Mn(HSal)2·2.5H2O
DTA曲线表明样品热分解有3个明显的吸热峰: 135℃为失去结晶水及H2O汽化的吸热峰; 220℃为水杨酸锰分解生成内盐和水杨酸以及水杨酸的升华吸热峰; 330℃是内盐分解生成MnO的吸热峰。
2.2 水杨酸锰的晶体结构
X射线粉末衍射数据表明样品中不存在碳酸锰和水杨酸。 用Powder X and UnitCell程序处理X射线粉末衍射数据, 结果表明Mn(HSal)2·2.5H2O的晶体属于单斜晶系, 晶胞参数为a=1.8935(4) nm, b=0.6179(0) nm, c=1.7325(6) nm, β=114.93(1)°, V=1.8382(3) nm3, Z=6, ρcal=1.775kg/dm3, ρexp=1.769kg/dm3; θ= 0.1°。 衍射数据的实验值和计算值比较吻合, 其中最强衍射峰(I/I1=100)在(100)晶面, 其余晶面的衍射强度都比较弱, 由此可以推测, Mn原子位于(100)晶面上, 苯环位于该晶面的两侧, 故该配合物为层状的晶体结构。
2.3 水杨酸锰的红外光谱分析
Mn(HSal)2·2.5H2O的红外光谱的主要振动波数及其归属如表1所列。 在2065和1655cm-1附近没有出现吸收峰, 表明样品中不存在自由羧基[13]。 在3491cm-1附近的强吸收峰是酚羟基的伸缩振动, 3421和3161cm-1处的强吸收峰是水的反对称和对称伸缩振动, 1539和1404cm-1为OCO的反对称和对称伸缩振动, 这表明羧酸根与Mn2+之间是以双齿螯合方式配位的[14]。
表1 样品红外光谱的主要振动波数及其归属
Table 1 Principal vibration wave number and assignment of IR spectrum for sample

2.4 热分解产物的表征
水杨酸锰在氮气气氛中经330℃热分解的固相残留物为灰绿色粉末, 其红外光谱在598和752cm-1处的振动吸收峰为Mn—O键的伸缩振动, 与MnO的标准图谱相一致[15]。 残留物的粉末X射线衍射谱如图2(a)所示, 计算得a=0.4438nm, 属立方晶系, 其数据与标准卡片JCPDS5—40439的数据相一致, 由此可以证明此固相残留物为MnO。
水杨酸锰在空气中经800℃热分解的固相残留物为灰色粉末。 其粉末X射线衍射谱示如图2(b)所示, 计算得a=0.5037nm, 属立方晶系, 其数据与Mn3O4标准卡片JCPDS24—734的数据一致, 由此可以证明此固相残留物是Mn3O4。
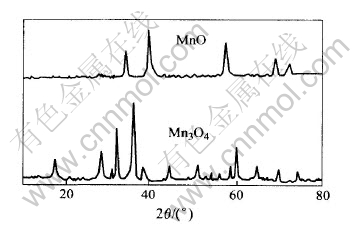
图2 MnO和Mn3O4的粉末X射线衍射谱
Fig.2 Powder X-ray diffraction pattern of MnO and Mn3O4
根据Scherrer公式计算MnO和Mn3O4的平均粒径分别为23.7和26.3nm。
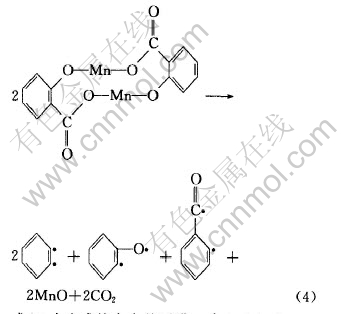
在氮气气氛中经330℃分解反应的气相凝聚物为淡红色粘稠物, 用丙酮浸泡, 其中不溶物是一种无色晶体, 其红外光谱(图3)与呫吨酮的标准红外光谱非常相似(IR, the Sadtler Standard Spectra. no. 18091K)。 将分离不溶物后的溶液在90℃干燥除去丙酮, 得到纯净的红棕色液体, 其色谱-质谱联用分析结果(表2)表明, 热分解气相凝聚物主要包含二苯并呋喃、 呫吨、 呫吨酮、 1, 2-二苯并吡喃、 2-苯基-1, 2-二苯并吡喃和1, 3-二苯基异苯并呋喃等。
图4所示为纳米MnO和Mn3O4在0.02~2000nm范围内的粒度分布曲线。 2种粒子的平均 粒径均在100nm以下且粒度分布范围较窄, 这与根据Scherrer公式计算得到的平均粒径有较大差别。 其原因是用激光粒度仪测MnO和Mn3O4的粒度分布时用水做分散剂, 由于2种粉体在水中发生弱团聚作用, 故用激光粒度仪测得的数据是MnO和Mn3O4在水中聚集体的平均粒径, 而Scherrer公式计算的是单个晶胞的平均粒径。
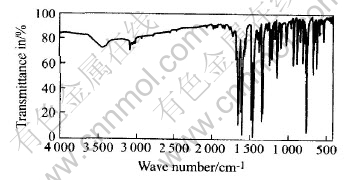
图3 呫吨酮(热分解产物)的红外光谱
Fig.3 IR spectra of xanthenone
(Thermal decomposition product)
表2 气相凝聚物色谱-质谱联用分析结果
Table 2 Analytical results of gas phase products chromatography-mass spectrometry(in N2)
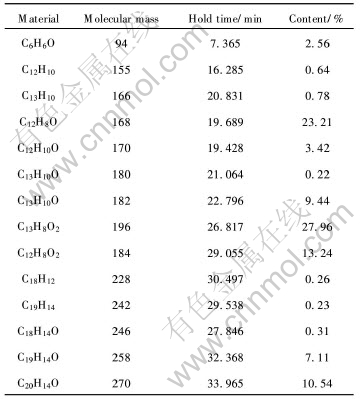
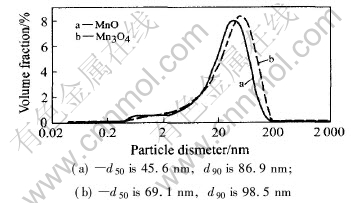
图4 氧化锰的粒度分布曲线
Fig.4 Size distribution curve of samples
图5所示为氮气气氛下经330℃热分解水杨酸锰固相残留物MnO和在空气中经800℃热分解水杨酸锰固相残留物Mn3O4的TEM照片。 由图可看出, MnO和Mn3O4基本上为球形粒子, 平均粒径分别为25和28nm, 与Scherrer公式计算的平均粒径非常接近。 由此可以说明用流变相-前驱物热分 解方法制备粒径较小的纳米粉体是完全可行的。

图5 MnO和Mn3O4的TEM照片
Fig.5 TEM photograph of MnO(a) and Mn3O4(b)
2.5 纳米粒子的生成机理
在用流变相-前驱物热分解方法制备纳米粒子的过程中, 经历了前驱物的成核、 生长、 洗涤、 干燥和煅烧等过程, 每个过程都可能产生粒子的团聚。 在成核、 生长过程中, 团聚的主要原因是颗粒表面存在非架桥羟基使颗粒之间发生缓慢团聚, 在干燥脱水过程中存在颗粒的毛细管收缩作用。 前驱物在高温热分解过程中, 粒子之间靠静电作用慢慢长大。 但在本文实验中, 采用乙醇洗涤前驱物减小了非架桥羟基的形成及脱水干燥过程中毛细管的收缩作用。 由于前驱物对乙醇的物理和化学吸附作用, 阻止了颗粒的靠近。 同时, 前驱物高温下热分解产生的气体也是防止粒子团聚的有效方法之一。
2.6 水杨酸锰的热分解机理
根据TG分析实验可以推测Mn(HSal)2·2.5H2O的热分解反应的机理如下:
有机化合物则按如下机理生成:
式(4)中生成的自由基可进一步生成如式(5)、 (6)、 (7)、 (8)和(9)所示的自由基和分子碎片。
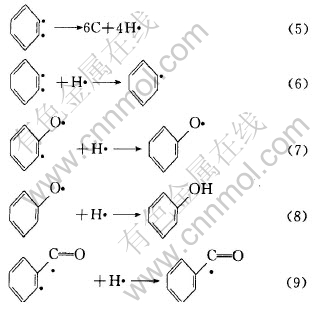
上述自由基可以相互结合生成如表2所示的二苯并呋喃、 呫吨、 呫吨酮、 1, 2-二苯并吡喃、 2-苯基-1, 2-二苯并吡喃和1, 3-二苯基异苯并呋喃等有机化合物。
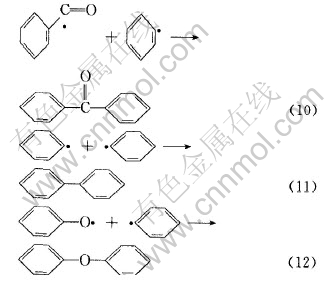
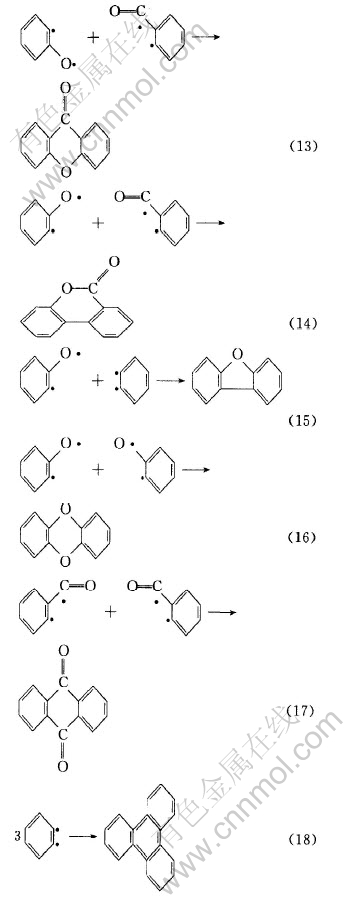
3 结论
1) 用流变相反应法合成了单斜晶系的Mn-(HSal)2·2.5H2O样品;
2) 样品在氮气中330℃热分解产物为纳米MnO粉末和有机化合物, 在空气中800℃热分解产物为纳米Mn3O4粉末;
3) TEM观察表明MnO和Mn3O4为球形粒子, 平均粒径分别为25和28nm;
4) 用流变相-前驱物热分解方法制备粒径较小的纳米粉体是完全可行的。
REFERENCES
[1]Heleua P S, Marc A A, Thomas W C. Sol-Gel-dived thin-film manganese dioxide cathodes[J]. J Electrochem Soc, 1996, 143(50): 1629-1632.
[2]李志光, 刘素琴, 黄可龙. 不同锰源合成尖金石型LixMn2O4及其性能[J]. 中国有色金属学报, 2003, 13(2): 526-529.
LI Zhi-guang, LIU Su-qin, HUANG Ke-long. Synthesis of spinel type LixMn2O4 and its properties[J]. The Chinese Journal of Nonferrous Metals, 2003, 13(2): 526-529.
[3]古映莹, 刘占明, 贾莉英, 等. 隐邻锰型水合MnO2的合成及其离子交换选择性[J]. 中国有色金属学报, 2001, 11(5): 915-919.
GU Ying-ying, LIU Zhan-ming, JIA Li-ying, et al. Synthesis of cryptomelane-type hydrous manganese dioxide and its ion-exchange[J]. The Chinese Journal of Nonferrous Metals, 2001, 11(5): 915-919.
[4]李志友, 曲选辉, 黄伯云. 化学锂化二氧化锰的高温电化学嵌锂行为[J]. 中国有色金属学报, 2000, 10(2): 203-208.
LI Zhi-you, QU Xuan-hui, HUANG Bai-yun. Electrochemical intercalation performance of chemically lithiated manganese dioxide at high temperature[J]. The Chinese Journal of Nonferrous Metals, 2000, 10(2): 203-208.
[5]刘玲, 夏熙. 纳米电极材料的制备及其电化学性质研究(Ⅲ): 微乳液中通O2制备MnO2及其性能[J]. 电化学, 1998, 4(3): 328-332.
LIU Ling, XIA Xi. Studies on the preparation of nanophase electrode material and its electrochemical properties Ⅲ: performance of nano-γ-MnO2 obtained by microemulsion method[J]. Electrochemisty, 1998, 4(3): 328-332.
[6]李娟, 李庆文, 夏熙. 纳米MnO2粉末的固相合成及其电化学性质研究[J]. 应用化学, 1999, 16(3): 103-105.
LI Juan, LI Qing-wen, XIA Xi. Synthesis of nanosize MnO2 by solid state reaction and its electrochemical property Ⅲ: synthesis and performance of α-MnO2[J]. Chinese Journal of Applied Chemistry, 1999, 16(3): 103-105.
[7]郭广生, 王志华, 李强, 等. 激光蒸凝法制备氧化锰纳米粒子[J]. 中国激光, 2002, A29(6): 553-538.
GUO Guang-sheng, WANG Zhi-hua, LI Qiang, et al. Preparation of MnO nanoparticles by laser heating gas evaporation method[J]. Chinese Journal of Lasers, 2002, A29(6): 553-538.
[8]李良超, 袁良杰, 杨毅涌, 等. 水杨酸镍配合物的合成和红外光谱研究[J]. 光谱学与光谱分析, 2000, 20(5): 671-672.
LI Liang-chao, YUAN Liang-jie, YANG Yi-yong, et al. Studies on synthesis and IR spectrum of nickel salicylate complexes[J] . Spectroscopy and Spectral Analysis, 2000, 20(5): 671-672.
[9]李良超, 李进, 袁良杰. 碱式水杨酸镍配合物的合成及性质研究[J]. 武汉大学学报(自然科学版), 2000, 化学专刊: 11-13.
LI Liang-chao, LI Jin, YUAN Liang-jie. Synthesis and characterization of nickel hydroxide salicylate complexes[J]. J of Wuhan Univ.(Nature Science Edition), 2000(S1): 11-13.
[10]李良超, 李自成, 袁良杰, 等. 水杨酸镧的流变相合成、 表征及掺杂Tb3+的水杨酸镧的发光性质[J]. 光谱学与光谱分析, 2004, 24(2): 142-145.
LI Liang-chao, LI Zi-cheng, , YUAN Liang-jie, et al. Rheological phase synthesis and characterization of lanthanum salicylate and luminescence properties of Tb3+-doped lanthanum salicylate[J]. Spectroscopy and Spectral Analysis, 2004, 24(2): 142-145.
[11]YANG Yi-yong, LI Liang-chao, YUAN Liang-jie, et al. Rheological phase synthesis and characterization of copper salicylate[J]. Wuhan Univ J of Nat Sci, 2003, 8(2A): 425-427.
[12]SUN J, Yuan L, ZHANG K, et al. The thermal decomposition mechanism of zinc phthalate[J]. Thermochimica Acta, 2000, 343: 105-109.
[13]Wlodzimierz L, Halina B. The influence of selected metals on the aromatic system of salicylic acid[J]. Applied Spectroscopy, 1987, 41(6): 976-980.
[14]中本一雄. 无机和配合物的红外和拉曼光谱(第四版)[M]. 黄德如, 译. 北京: 化学工业出版社, 1991. 257.
Nakamoto K. Infrared and Raman Spectra of Inorganic and Coordination Compounds[M]. (4 Ed.). HUANG De-ru, tran. Beijing: Chemical Industry Press, 1991. 257.
[15]Richard A N, Ronald O K. Infrared Spectra of Inorganic Compounds[M]. New Work and London: Academic Press, 1971. 217.
(编辑李艳红)
基金项目: 浙江师范大学无机化学重点扶持学科基金资助项目(ZC319003230); 引进人才基金资助项目(KYJ03Y02023)
收稿日期: 2004-07-06; 修订日期: 2004-09-21
作者简介: 李良超(1958-), 男, 教授.
通讯作者: 李良超, 浙江金华市浙江师范大学60#信箱; 电话: 0579-2283088; E-mail: lilc58715@sina.com.cn