一种用于嗜酸性氧化亚铁硫杆菌液氮保藏的新型保护剂的保藏效果
来源期刊:中国有色金属学报(英文版)2013年第3期
论文作者:吴学玲 胡 琪 侯冬梅 辛小红 苗 博 王洋洋 刘学端 申 丽
文章页码:818 - 823
关键词:嗜酸性氧化亚铁硫杆菌;冷冻保护剂;液氮保藏;保藏效率
Key words:Acidithiobacillus ferrooxidans; cryoprotectant; liquid nitrogen freezing; preservation efficiency
摘 要:对一种用于嗜酸性氧化亚铁硫杆菌液氮冷藏新型保护剂GP的保藏效果进行研究。依据最大细胞复苏率及最高亚铁氧化活性确定该新型保护剂的最佳使用浓度。结果表明,保护剂的最佳浓度为30%,在此浓度下细胞复苏率达到84.4%,且能在120 h内完全氧化培养基中的亚铁,培养6 d后菌体浓度达到5.8×107 cell/mL。此外,解冻细胞在9K培养基中培养6 d后,对活细胞复苏的最佳GP残留浓度为0.6%(体积分数)。在此浓度下,菌株DC完全氧化亚铁需要108 h,并且最终菌体浓度为6.8×107 cell/mL. 因此,GP是一种简单、有效的嗜酸性氧化亚铁硫杆菌液氮保藏的冷冻保护剂。
Abstract: The efficiency of a new cryoprotectant, GP, for the preservation of Acidithiobacillus ferrooxidans (A. ferrooxidans) strain DC in liquid nitrogen was investigated. The optimal concentration of this new cryoprotectant for the maximal viable cell recovery and the highest ferrous ion oxidation activity was determined. The results show that 30% (volume fraction) GP is optimal for the cryopreservation with 84.4% of cells surviving, completely oxidizing ferrous ions within 120 h, and growing to a final density of 5.8×107 cell/mL after 6 d in the culture. Furthermore, the optimal residual GP concentration for viable cell recovery after culture of thawed cells in 9K medium for 6 d is 0.6% (volume fraction). At this concentration, strain DC completely oxidizes ferrous ions within 108 h and grows to a final cell density of 6.8×107 mL-1. Thus, GP is a simple, effective cryoprotectant for the preservation of A. ferrooxidans strain DC in liquid nitrogen.
Trans. Nonferrous Met. Soc. China 23(2013) 818-823
Xue-ling WU1, Qi HU1, Dong-mei HOU1, Xiao-hong XIN2, Bo MIAO1, Yang-yang WANG1, Xue-duan LIU1, Li SHEN1
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. China Center for Type Culture Collection, College of Life Sciences, Wuhan University, Wuhan 430072, China
Received 5 November 2011; accepted 12 October 2012
Abstract: The efficiency of a new cryoprotectant, GP, for the preservation of Acidithiobacillus ferrooxidans (A. ferrooxidans) strain DC in liquid nitrogen was investigated. The optimal concentration of this new cryoprotectant for the maximal viable cell recovery and the highest ferrous ion oxidation activity was determined. The results show that 30% (volume fraction) GP is optimal for the cryopreservation with 84.4% of cells surviving, completely oxidizing ferrous ions within 120 h, and growing to a final density of 5.8×107 cell/mL after 6 d in the culture. Furthermore, the optimal residual GP concentration for viable cell recovery after culture of thawed cells in 9K medium for 6 d is 0.6% (volume fraction). At this concentration, strain DC completely oxidizes ferrous ions within 108 h and grows to a final cell density of 6.8×107 mL-1. Thus, GP is a simple, effective cryoprotectant for the preservation of A. ferrooxidans strain DC in liquid nitrogen.
Key words: Acidithiobacillus ferrooxidans; cryoprotectant; liquid nitrogen freezing; preservation efficiency
1 Introduction
Presently, bioleaching is the leading mineral processing technique by which metals are dissolved from ores into solution through oxidation and decomposition processes of the ores by microorganisms, and the dissolved metals are finally extracted from solution as pure metals by electrochemical methods [1,2]. It has advantages over conventional methods for extracting metals from low-grade, complicated ores [3]. Bioleaching bacteria play an important role in these processes [4,5]. Acidithiobacillus ferrooxidans (A. ferrooxidans) is commonly used to extract copper, lead, zinc, uranium, gold, and nickel from the insoluble sulfide minerals of these metals [6,7]. Therefore, methods are needed to maintain and preserve these microorganisms and maximize their activities.
Cryopreservation of bioleaching bacteria was first reported about 35 years ago by MANCHEE [8], and methods, protocols, and cryoprotectants for these organisms were subsequently developed [9]. ZENG et al [10] examined four different methods to store the bioleaching bacterium Acidithiobacillus caldus S2 (A. caldus) and found that A. caldus strain S2 could be stored short term in tubes containing sterile sand, and in long term (15 months) by freeze drying. Unfortunately, the survival rate of A. caldus strain S2 was only 17% after 15 months storage. CLELAND et al [11] noted the sensitivity of the genus Acidithiobacillus to freeze-dry preservation, although this method of culture preservation is commonly employed by culture collections. In their experiments, mono-sodium- glutamate and glycine betaine were used as cryopreservatives. Nevertheless, only 0-104 cell/mL survived cryostorage, and the recovery was dependent on both the cryoprotectant and the medium.
Other successful methods of long-term preservation include freezing in liquid nitrogen at -70 °C [12], or freezing in different cryoprotectants [13,14]. Liquid nitrogen freezing [15] is generally thought to cause less cellular damage, and many strains have been stored in long term by this method. However, few reports address the problem of liquid nitrogen preservation of mesophilic and acidophilic ferrous ion-oxidizing bacteria. The protective agent is critical. CLELAND et al [16] found that Fe-oxidizing bacteria survived longer in glycerol than in glycine betaine. In our laboratory, 30% glycerol successfully cryoprotected the mesophilic and acidophilic ferrous ion-oxidizing bacterium A. ferrooxidans DC in liquid nitrogen storage [12]. However, the activities of A. ferrooxidans are often inhibited by organic compounds [17] such as milk and glycerol. So, after retrieval from storage, the cells must be centrifuged to remove the glycerol, washed twice with sterile distilled water (pH 2), and transferred twice into fresh medium to promote the recovery after immersion in liquid nitrogen. Although capable of preserving A. ferrooxidans in liquid nitrogen, glycerol is not an ideal cryoprotectant for many bioleaching bacteria because of the complicated retrieval procedure.
GP is a hydrophic nontoxic molecule, with properties similar to other compounds used as cryoprotectants for prokaryotic cells during freezing [16]. Therefore, in this work, GP was used as cryoprotectant of A. ferrooxidans DC during liquid nitrogen freezing. The effectiveness of this agent as a cryopreservative agent was investigated and its optimal concentration was determined.
2 Experimental
2.1 Microorganisms and growth conditions
The mesophilic and acidophilic ferrous ion- oxidizing bacterium, A. ferrooxidans strain DC, was isolated and maintained in the laboratory [18] in 9K medium [11], which was designed for Fe-reducers. 9K medium contained 3 g/L (NH4)2SO4, 3 g/L KH2PO4, 0.5 g/L MgSO4·7H2O, 0.25 g/L CaCl2·2H2O, and 30 g/L FeSO4·7H2O. The cultured medium was autoclaved at 121 °C for 30 min, and the bacteria were cultured in a shaking incubator (180 r/min) at 30°C. The pH value of the 9K medium was adjusted to 2.0 by adding half- concentrated H2SO4 before the addition of FeSO4·7H2O.
2.2 Cryoprotective agent
The cryoprotective agent used in this work was GP, which was provided by the China Center for Type Culture Collection (CCTCC). GP was diluted into a series of concentrations in deionized water (d-H2O) and the series was sterilized by autoclaving.
2.3 Cell preservation
The cells of A. ferrooxidans DC were harvested during late logarithmic phase. The cultures were vigorously hand-shaken for 1 min and allowed to sit at room temperature for 30 min to allow the particulates of iron oxides to settle. The culture fluid was transferred to another fresh sterile tube (leaving large particulates behind) and centrifuged at 10000 r/min and 4 °C for 15 min to pellet the cells.
In the preliminary experiments, different GP concentrations (10%-100%, volume fraction) were tested and the results showed that at GP concentration from 10% to 50% cells could be retrieved from storage using GP as the cryopreservative agent (data are not shown). So in present work, the cell pellet was suspended in sterile distilled water (pH 6.8) [19], and distributed into ampoules with sterile distilled water containing either GP 10%, 20%, 30%, 40%, or 50% (volume fraction). Triplicate ampoules were made for each GP concentration.
2.4 Storage in liquid nitrogen and retrieval from liquid nitrogen
Firstly, the ampoules of bacteria were placed at 4 °C for 30-40 min, transferred to -20 °C for 1 h, transferred to -70 °C for 2 h, and finally placed in a liquid nitrogen freezer for 3 months.
The ampoules were retrieved from liquid nitrogen after 3 months, immediately thawed in a water-bath at 37 °C for 10 min, and the contents were transferred to fresh medium.
2.5 Determination of optimum concentration of GP for cryopreservation in liquid nitrogen
After the retrieval of the cells, the survival rates, final cell densities, and ferrous ion oxidation rates were determined to assess the optimal concentration of GP.
2.5.1 Cell survival rate
Samples from fresh cultures and the thawed ampoules were inoculated in fresh medium to assess the survival rates of A. ferrooxidans DC. The cell survival rate (η) was calculated from the cell density before preservation (N0) and cell density after preservation (Nt) according to:
η=Nt/N0×100% (1)
2.5.2 Final cell density
After being inoculated in fresh medium, the bacteria were cultured for 6 d, and then the final cell density was counted by light microscopy.
2.5.3 Ferrous iron oxidation rate
To determine the ferrous oxidation activity of A. ferrooxidans DC after storage in different concentrations of GP, the ferrous ion concentration in 9K medium was assayed at 12-h intervals for a period of 144 h by the K2Cr2O7 titration method [1].
2.6 Optimal residual concentration of GP for cells retrieved from liquid nitrogen
After determining the optimal concentration of GP for cell preservation, the experiments were carried out to determine the residual concentration of GP optimal for the growth and activity of cells retrieved from liquid nitrogen. After being retrieved, the samples were added to fresh 9K medium, during which GPs were diluted into a series of concentrations (0.2%, 0.4%, 0.6%, 0.8% and 1.0% (volume fraction) in 9K medium. GP with concentrations of 0.2%, 0.4%, 0.6%, 0.8% and 1.0% (volume fraction) in 9K medium were added to samples retrieved from liquid nitrogen. The optimal concentration of GP was determined by the assessment of the cell density and the ferrous ion oxidation rate of cells cultured from these samples.
All experiments were repeated in triplicate. Unpreserved cells (cells exposed to cryopreservative agent) were used as the control, and aseptic culture medium was used as the blank.
3 Results
3.1 Determination of optimal GP concentration for A. ferrooxidans DC cryopreservation in liquid nitrogen
3.1.1 Cell survival rate
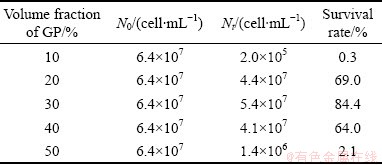
The survival rates of A. ferrooxidans DC stored for 3 months in liquid nitrogen and thawed immediately are high, which are 69%, 84.4%, and 64%, at GP concentrations of 20%, 30%, and 40%, respectively, as shown in Table 1. The drop in viability after liquid nitrogen freezing was only 0.1–0.2 log10 (Meaning of log10 refers to Ref. [11]). However, only 0.3% and 2.1% of cells survived after storage in GP with concentrations of 10% and 50%, respectively.
Table 1 Survival rates of A. ferrooxidans DC after cryopreservation in different concentrations of GP
3.1.2 Final cell density
The densities of A. ferrooxidans DC cultured for 6 d in fresh medium following storage in different GP concentrations are shown in Fig. 1. The values given are the mean of three samples enumerated. In all cases the standard deviation about the mean is less than 5% of the mean. The final cell densities after preservation in different concentration of GP (10%, 20%, 30%, 40%, and 50%) are 2.1×106, 5.2×107, 5.8×107, 4.8×107, and 1.2×107 cell/mL, respectively. The density of cultured cells from samples containing the most effective GP concentration (30%) is almost 0.04-0.08 log10 higher than that of cells from samples containing 20% GP and 40% GP and almost (0.5-1.5) log10 higher than that of cells from samples containing 10% GP and 50% GP. Based on the survival rates and the final cell densities, cells preserved in 20% GP, 30% GP, and 40% GP were chosen for the assay of the ferrous ion oxidation rate.
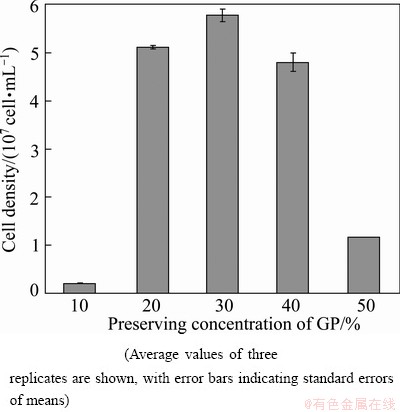
Fig. 1 Cell density of A. ferrooxidans DC cryopreserved in different concentrations of GP, retrieved from liquid nitrogen, and cultured for 6 d in 9K medium
3.1.3 Ferrous ion oxidation rate
The ferrous ion oxidation rate of A. ferrooxidans strain DC was assayed after retrieval from cryostorage (Fig. 2). In the control (culture of cells that were not exposed to GP), ferrous ions were completely oxidized within 96 h. However, in cultures of cells after freezing with 30% GP and 20% GP, ferrous ions were completely oxidized within 120 and 144 h, respectively. Only 90.1% ferrous ions were oxidized in 144 h by cells exposed to 40% GP.
3.2 Determination of residual concentration of GP for optimal cell retrieval
Some preliminary experiments (data not shown) indicated that the concentration of GP in 9K medium influenced the cell retrieval. According to Ref. [12], residual cryoprotective organic agents had this effect [12]. So, experiments were carried out to determine the optimal residual concentration of GP in cultures of the retrieved samples. The cultures of cells stored in 30% GP (the optimal concentration for storage) were used in these experiments. The residual concentration of GP in 9K medium (after retrieval of cells cryopreserved in 30% GP and transfer to 9K medium) was almost 0.48% (volume fraction).
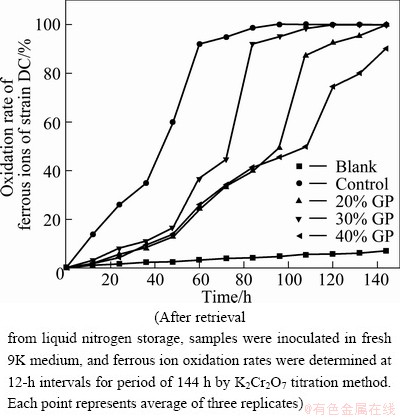
Fig. 2 Ferrous ion oxidation rates of A. ferrooxidans DC cryopreserved in different concentrations of GP
After cells preserved in 30% GP were exposed to GP (0.2%, 0.4%, 0.6%, 0.8%, and 1.0%, volume fraction) in 9K medium and cultured for 6 d, cell densities and ferrous ion oxidation rates were measured.
3.2.1 Final cell density
The cell density is maximal (6.8×107 cell/mL) when the residual concentration of GP is 0.6% (volume fraction). At other residual concentrations, the cell densities are lower than 4.5×107 cell/mL at 0.4% GP, 3.13×107 cell/mL at 0.2% GP, 3.5×107 cell/mL at 0.8% GP, and 1.85×107 cell/mL at 1.0% GP (Fig. 3).
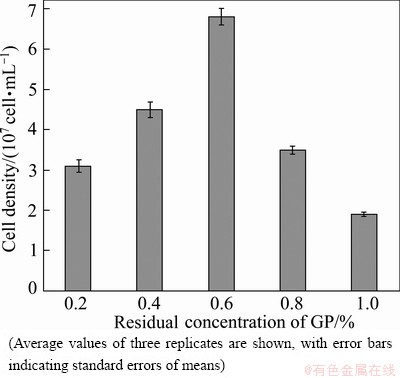
Fig. 3 Cell density of A. ferrooxidans DC after culture in 9K medium containing different residual GP concentrations for 6 d
3.2.2 Ferrous ion oxidation rate
At residual concentrations of 0.6% GP and 0.4% GP, ferrous ions are completely oxidized within 108 and 132 h, respectively. At residual concentrations of 0.2% GP, 0.8% GP, and 1.0% GP, only 98.6%, 80.3%, and 70.1%, respectively, of ferrous ions were oxidized within 144 h (Fig. 4). These results confirm that 0.6% GP residual concentration in 9K medium is optimal for the retrieval of A. ferrooxidans DC cells cryopreserved with GP in liquid nitrogen.
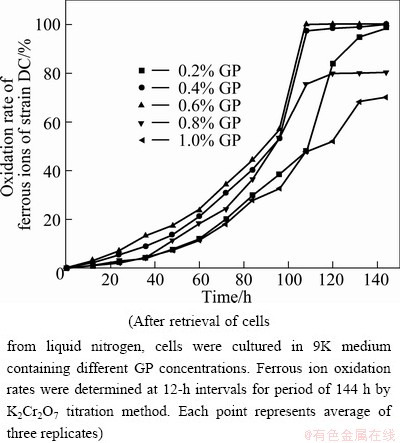
Fig. 4 Ferrous ion oxidation rates of A. ferrooxidans DC in different residual concentrations of GP
4 Discussion
Liquid nitrogen freezing is the preferred method of long-term preservation of microorganisms. But A. ferrooxidans is highly susceptible to cryoinjury. The loss of cell viability during freezing is probably due to the damage of cell membranes and nucleic acids [20]. A suitable cryoprotectant is very important in reducing the extent of this damage [20-24], and the presence of a suitable cryoprotective agent (CPA) usually increases the survival considerably [14]. On the other hand, lyoprotectants such as skimmed milk, serum, and sugars, inhibit and therefore are not suitable for CPAs for A. ferrooxidans cryopreservation. In the present work, GP as a cryopreservative of A. ferrooxidans DC in liquid nitrogen was evaluated.
After liquid nitrogen freezing, the survival rate at 30% GP is higher (84.4%, only approximately 0.16 log10 loss of cells during the freezing process) than at other GP concentrations. CLELAND et al [11] using glycine betaine, another reagent used for A. ferrooxidans freeze dry preservation, found a (1-2) log10 loss of viability after the preservation. Thus, compared with glycine betaine, GP is a more effective preservative of A. ferrooxidans during storage.
Ferrous ion oxidation is important in evaluating the growth of A. ferrooxidans. Thus, in this work, the oxidation rates of ferrous ions were measured to evaluate the activity of these bacteria after retrieval from storage. It is shown that the optimal concentration of GP for cryopreservation of A. ferrooxidans DC is 30%. At this concentration, cells completely oxidize ferrous ions within 120 h (shorter than that at 20% GP or 40% GP) and the density of cells cultured for 6 d is 5.8×107 cell/mL, which is higher than those at other concentrations.
However, the growth rate of these bacteria is found to decrease after freezing. This is consistent with the reported findings [10] that bioleaching microorganisms take longer to adapt to the growth environment after preservation.  [22] suggested that cell damage during the preservation or toxicity of the CPA accounts for this delay in recovery. Hence, many CPAs should be removed by centrifugation or dilution after thawing. For instance, the dimethylsulfoxide (Me2SO) concentration should be lowered to 0.35% (volume fraction) in suspensions of most eukaryotic cells [22] and glycerol must be removed after retrieval of A. ferrooxidans from cryostorage [12].
[22] suggested that cell damage during the preservation or toxicity of the CPA accounts for this delay in recovery. Hence, many CPAs should be removed by centrifugation or dilution after thawing. For instance, the dimethylsulfoxide (Me2SO) concentration should be lowered to 0.35% (volume fraction) in suspensions of most eukaryotic cells [22] and glycerol must be removed after retrieval of A. ferrooxidans from cryostorage [12].
Many procedures to reduce cryodamage in A. ferrooxidans were proposed. For example, WU et al [12] proposed removing the glycerol and transferring cells twice into fresh media to obtain good growth. More recent studies by ZENG et al [10] suggested prolonging time of A. ferrooxidans in the culture to promote the recovery of good growth and biological activity. In this work, it is not necessary to remove GP or prolong the time in culture after the retrieval from the storage. Dilution in 9K medium down to a residual GP concentration of 0.6% is sufficient to maximize the viability and activity of A. ferrooxidans DC retrieved from cryostorage. At this concentration, ferrous ions are completely oxidized within 108 h, and after 6 d in culture cells reach a final density of 6.8×107 cell/mL, which is similar to the density achieved in culture by unpreserved cells (i.e., cells that were not exposed to cryopreservative agent; 6.4×107 cell/mL). GP might play an important role in the cell damage repair and recovery of activity, or serve as a nutrient or growth promoter for A. ferrooxidans. However, there are GP concentrations that are harmful. Residual concentrations of GP higher than 0.6% decrease the cell viability and activity. This finding provides support for the view that residual concentrations of cryoprotectants can be optimized [24,25].
The current work is apparently the first to use GP as a cryoprotectant to preserve a bioleaching microorganism in liquid nitrogen storage. The results demonstrate that GP can cryoprotect A. ferrooxidans DC in liquid nitrogen storage for a long time. Like all cryopreservative agents, GP should be tested empirically to determine whether its use for a novel group of microorganisms is appropriate. GP as a cryopreservative agent for other species of Acidothiobacillus as well as other extremophiles will be tested further.
5 Conclusions
1) 30% GP is the optimal concentration for preserving A. ferrooxidans DC in liquid nitrogen cryostorage.
2) The optimal residual concentration of GP is 0.6% in 9K medium. At this concentration, the cell growth and ferrous ion oxidation activity in A. ferrooxidans DC are good.
References
[1] WU X L, QIU G Z, GAO J, DING J N, KANG J, LIU X X. Mutagenic breeding of silver-resistant Acidithiobacillus ferrooxidans and exploration of resistant mechanism [J]. Transactions of Nonferrous Metals Society of China, 2007, 17: 412-417.
[2] YIN H Q, QIU G Z, LUO H L, CAO L H, DAI Z M, WANG J W, WANG D Z, LIU X D. The research of ferrous iron-oxidizing capability of Acidithiobacillus ferrooxidans and the efficiency of low grade chalcopyrite bioleaching [J]. Progr Mod Biomed, 2007, 7: 641-642.
[3] CABRERA G,  J M, CANTERO D. Kinetic study of ferrous sulphate oxidation of Acidithiobacillus ferrooxidans in the presence of heavy metal ions [J]. Enzyme Microb Tech, 2005, 36: 301-306.
J M, CANTERO D. Kinetic study of ferrous sulphate oxidation of Acidithiobacillus ferrooxidans in the presence of heavy metal ions [J]. Enzyme Microb Tech, 2005, 36: 301-306.
[4] HALLBERG K B, JOHNSON D B. Novel acidophiles isolated from moderately acidic mine drainage waters [J]. Hydrometallurgy, 2003, 71: 139-148.
[5] WATLING H R. The bioleaching of sulphide minerals with emphasis on copper sulphides—A review [J]. Hydrometallurgy, 2006, 84: 81-108.
[6] SARCHESHMEHPOUR Z, LAKZIAN A, FOTOVAT A, BERENJI A R, HAGHNIA G H, BAGHERI S A S. Possibility of using chemical fertilizers instead of 9K medium in bioleaching process of low-grade sulfide copper ores [J]. Hydrometallurgy, 2009, 96: 264-267.
[7] RAWLINGS D, KUSANO T. Molecular genetics of Thiobacillus ferrooxidans [J]. Microbiol Rev, 1994, 58: 39-55.
[8] MANCHEE R J. Long term storage of Thiobacillus ferrooxidans [J]. J Appl Bact, 1975, 38: 191-192.
[9] WATKIN E L J,KEELING S E,PERROT F A,SHIERS D W,PALMER M L,WATLING H R. Metals tolerance in moderately thermophilic isolates from a spent copper sulfide heap, closely related to Acidithiobacillus caldus, Acidimicrobium ferrooxidans and Sulfobacillus thermosulfidooxidans [J]. J Ind Microbiol Biot, 2009, 36: 461-465.
[10] ZENG W M, ZHOU H B, WAN M X, CHAO W L, XU A L, LIU X D, QIU G Z. Preservation of Acidithiobacillus caldus: A moderately thermophilic bacterium and the effect on subsequent bioleaching of chalcopyrite [J]. Hydrometallurgy, 2009, 96: 333-336.
[11] CLELAND D, KRADER P, TANG J, EMERSON D. Preservation of Acidithiobacillus by liquid-drying using glycine betaine as the cryoprotectant [C]//Proceedings of 101st General Meeting for American Society for Microbiology. Orlando, 2001, 418.
[12] WU X L, XIN X H, JIANG Y, LIANG R X, YUAN P, FANG C X. Liquid-nitrogen cryopreservation of three kinds of autotrophic bioleaching bacteria [J]. Transactions of Nonferrous Metals Society of China, 2008, 18: 1386-1391.
[13] YANG Y, ZHANG Y F, HUANG J F, JIANG D H, CHEN Y, QIU G Z. Preservation of Acidithiobacillus ferrooxidans [J]. Journal of Central South University: Science and Technology, 2006, 37: 472-475. (in Chinese)
[14] YANG Y, ZHANG Y F, HE H, WAN M X, SHI W Y, QIU G Z. Optimization of frozen storage cryoprotectant for Acidithiobacillus ferroxidans by orthogonal experiment [J]. Journal of Central South University, 2006, 37: 891-895. (in Chinese)
[15] PALMFELDT J,  B. Optimisation of initial cell concentration enhances freeze-drying tolerance of Pseudomonas chloroaphis [J]. Cryobiology, 2003, 47: 21-29.
B. Optimisation of initial cell concentration enhances freeze-drying tolerance of Pseudomonas chloroaphis [J]. Cryobiology, 2003, 47: 21-29.
[16] CLELAND D, KRADER P, MCCREE C, TANG J, EMERSON D. Glycine betaine as a cryoprotectant for prokaryotes [J]. J Microbiol Meth, 2004, 58: 31-38.
[17] TUOYINEN O H, KELLY D P. Studies on the growth of Thiobacillus ferrooxidans. I. Use of membrane filters and ferrous iron agar to determine viable numbers, and comparison with 14CO2-fixation and iron oxidation as measures of growth [J]. Arch Microbiol, 1973, 88: 285-298.
[18] GUPTA S D, AGATE A D. Preservation of Thiobacillus ferrooxidans and Thiobacillus thiooxidans with activity check [J]. Anton Leeuw Int J G, 1986, 52: 121-127.
[19] ZHOU J K, QIU G Z, NIU Y J, QIN W Q. Effect of dryness preservation on Fe2+ oxidation activity of Thiobacillus of ferroxidation [J]. Journal of Central South University, 2004, 35: 39-42. (in Chinese)
[20] PETER G, REICHART O. The effect of growth phase, cryoprotectants and freezing rates on the survival of selected micro-organisms during freezing and thawing [J]. Acta Aliment Hung, 2001, 30: 89-97.
[21] PORTNER D C, LEUSCHNER R G K, MURRAY B S. Optimising the viability during storage of freeze-dried cell preparations of Campylobacter jejuni [J]. Cryobiology, 2007, 54: 265-270.
[22] HUBALEK Z. Protectants used in the cryopreservation of microorganisms [J]. Cryobiology, 2003, 46: 205-229.
[23] VUTHIPHANDCHAI V, CHOMPHUTHAWACH S, NIMRAT S. Cryopreservation of red snapper (Lutjanus argentimaculatus) sperm: Effect of cryoprotectants and cooling rates on sperm motility, sperm viability, and fertilization capacity [J]. Theriogenology, 2009, 72: 129-138.
[24] SAKANE T,
[25] SON J H, HEO Y J, PARK M Y, KIM H H, LEE K S. Optimization of cryopreservation condition for hematopoietic stem cells from umbilical cord blood [J]. Cryobiology, 2010, 60: 287-292.
吴学玲1, 胡 琪1,侯冬梅1,辛小红2,苗 博1,王洋洋1,刘学端1,申 丽1
1. 中南大学 资源加工与生物工程学院,长沙 410083;
2. 武汉大学 生命科学院,中国典型培养物保藏中心,武汉 430072
摘 要:对一种用于嗜酸性氧化亚铁硫杆菌液氮冷藏新型保护剂GP的保藏效果进行研究。依据最大细胞复苏率及最高亚铁氧化活性确定该新型保护剂的最佳使用浓度。结果表明,保护剂的最佳浓度为30%,在此浓度下细胞复苏率达到84.4%,且能在120 h内完全氧化培养基中的亚铁,培养6 d后菌体浓度达到5.8×107 cell/mL。此外,解冻细胞在9K培养基中培养6 d后,对活细胞复苏的最佳GP残留浓度为0.6%(体积分数)。在此浓度下,菌株DC完全氧化亚铁需要108 h,并且最终菌体浓度为6.8×107 cell/mL. 因此,GP是一种简单、有效的嗜酸性氧化亚铁硫杆菌液氮保藏的冷冻保护剂。
关键词:嗜酸性氧化亚铁硫杆菌;冷冻保护剂;液氮保藏;保藏效率
(Edited by Wei-ping CHEN)
Foundation item: Project (2005DKA21208) supported by the R&D Infrastructure and Facility Development Program from the Ministry of Science and Technology of China; Project (2010CB630901) supported by the National Basic Research Program of China
Corresponding author: Li SHEN; Tel: +86-731-88879815; E-mail: xiaolis@yahoo.cn
DOI: 10.1016/S1003-6326(13)62534-0

